Allogeneic cell therapy bioprocess economics and optimization: single-use cell expansion technologies
- PMID: 23893544
- PMCID: PMC4065358
- DOI: 10.1002/bit.25008
Allogeneic cell therapy bioprocess economics and optimization: single-use cell expansion technologies
Abstract
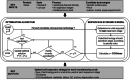
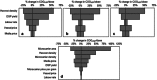
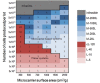
For allogeneic cell therapies to reach their therapeutic potential, challenges related to achieving scalable and robust manufacturing processes will need to be addressed. A particular challenge is producing lot-sizes capable of meeting commercial demands of up to 10(9) cells/dose for large patient numbers due to the current limitations of expansion technologies. This article describes the application of a decisional tool to identify the most cost-effective expansion technologies for different scales of production as well as current gaps in the technology capabilities for allogeneic cell therapy manufacture. The tool integrates bioprocess economics with optimization to assess the economic competitiveness of planar and microcarrier-based cell expansion technologies. Visualization methods were used to identify the production scales where planar technologies will cease to be cost-effective and where microcarrier-based bioreactors become the only option. The tool outputs also predict that for the industry to be sustainable for high demand scenarios, significant increases will likely be needed in the performance capabilities of microcarrier-based systems. These data are presented using a technology S-curve as well as windows of operation to identify the combination of cell productivities and scale of single-use bioreactors required to meet future lot sizes. The modeling insights can be used to identify where future R&D investment should be focused to improve the performance of the most promising technologies so that they become a robust and scalable option that enables the cell therapy industry reach commercially relevant lot sizes. The tool outputs can facilitate decision-making very early on in development and be used to predict, and better manage, the risk of process changes needed as products proceed through the development pathway.
Keywords: allogeneic cell therapy manufacture; bioprocess economics; cell factories; microcarriers; single-use cell expansion; stem cells.
© 2013 Wiley Periodicals, Inc.
Figures


References
-
- Birch JR, Racher AJ. Antibody production. Adv Drug Deliver Rev. 2006;58:671–685. - PubMed
-
- Bowden MJ. Moore's Law and the technology S-curve. Curr Issues Technol Manage. 2004;8(1):1–4.
-
- Brandenberger R, Burger S, Campbell A, Fong T, Lapinskas E, Rowley JA. Cell therapy bioprocessing. BioProcess Int. 2011;9(Suppl. 1):30–37.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

