Transcriptome profiling of Nasonia vitripennis testis reveals novel transcripts expressed from the selfish B chromosome, paternal sex ratio
- PMID: 23893741
- PMCID: PMC3755920
- DOI: 10.1534/g3.113.007583
Transcriptome profiling of Nasonia vitripennis testis reveals novel transcripts expressed from the selfish B chromosome, paternal sex ratio
Abstract
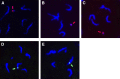
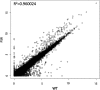
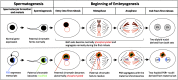
A widespread phenomenon in nature is sex ratio distortion of arthropod populations caused by microbial and genetic parasites. Currently little is known about how these agents alter host developmental processes to favor one sex or the other. The paternal sex ratio (PSR) chromosome is a nonessential, paternally transmitted centric fragment that segregates in natural populations of the jewel wasp, Nasonia vitripennis. To persist, PSR is thought to modify the hereditary material of the developing sperm, with the result that all nuclear DNA other than the PSR chromosome is destroyed shortly after fertilization. This results in the conversion of a fertilized embryo--normally a female--into a male, thereby insuring transmission of the "selfish" PSR chromosome, and simultaneously leading to wasp populations that are male-biased. To begin to understand this system at the mechanistic level, we carried out transcriptional profiling of testis from WT and PSR-carrying males. We identified a number of transcripts that are differentially expressed between these conditions. We also discovered nine transcripts that are uniquely expressed from the PSR chromosome. Four of these PSR-specific transcripts encode putative proteins, whereas the others have very short open reading frames and no homology to known proteins, suggesting that they are long noncoding RNAs. We propose several different models for how these transcripts could facilitate PSR-dependent effects. Our analyses also revealed 15.71 MB of novel transcribed regions in the N. vitripennis genome, thus increasing the current annotation of total transcribed regions by 53.4%. Finally, we detected expression of multiple meiosis-related genes in the wasp testis, despite the lack of conventional meiosis in the male sex.
Figures
References
-
- Adams M. D., Celniker S. E., Holt R. A., Evans C. A., Gocayne J. D., et al. , 2000. The genome sequence of Drosophila melanogaster. Science 287: 2185–2195 - PubMed
-
- Amrein H., Axel R., 1997. Genes expressed in neurons of adult male Drosophila. Cell 88: 459–469 - PubMed
-
- Bozzetti M. P., Massari S., Finelli P., Meggio F., Pinna L. A., et al. , 1995. The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl. Acad. Sci. USA 92: 6067–6071 - PMC - PubMed
-
- Brown C. J., Ballabio A., Rupert J. L., Lafreniere R. G., Grompe M., et al. , 1991. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature 349: 38–44 - PubMed
-
- Bull J. J., 1981. Coevolution of haplo-diploidy and sex determination in the hymenoptera. Evolution 35: 568–580 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
