Fatty acid remodeling in cellular glycerophospholipids following the activation of human T cells
- PMID: 23894206
- PMCID: PMC3770080
- DOI: 10.1194/jlr.M037044
Fatty acid remodeling in cellular glycerophospholipids following the activation of human T cells
Abstract
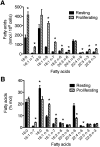
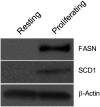
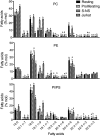
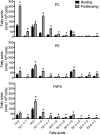
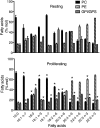
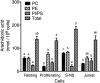
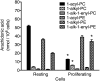
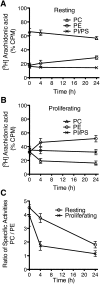
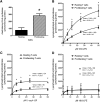
Changes in fatty acid (FA) and glycerophospholipid (GPL) metabolism associated with cell cycle entry are not fully understood. In this study FA-GPL remodeling was investigated in resting and proliferating primary human T cells. Significant changes were measured in the composition and distribution of FAs in GPLs following receptor activation of human T cells. The FA distribution of proliferating T cells was very similar to that of the human Jurkat T cell line and when the stimulus was removed from proliferating T cells, they stopped proliferating and the FA distribution largely reverted back to that of resting T cells. The cellular content of saturated and monounsaturated FAs was significantly increased in proliferating cells, which was associated with an induction of FA synthase and stearoyl-CoA desaturase-1 gene expression. Additionally, cellular arachidonate was redistributed in GPLs in a distinct pattern that was unlike any other FAs. This redistribution was associated with an induction of CoA-dependent and CoA-independent remodeling. Accordingly, significant changes in the expression of several acyl-CoA synthetases, lysophospholipid acyltransferases, and phospholipase A2 were measured. Overall, these results suggest that metabolic pathways are activated in proliferating T cells that may represent fundamental changes associated with human cell proliferation.
Keywords: acyl-CoA synthetase; arachidonic acid; fatty acid synthase; lysophospholipid acyltransferase; phospholipase A2; stearoyl-CoA desaturase 1.
Figures



References
-
- Spector A. A., Yorek M. A. 1985. Membrane lipid composition and cellular function. J. Lipid Res. 26: 1015–1035 - PubMed
-
- Yamashita A., Sugiura T., Waku K. 1997. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J. Biochem. 122: 1–16 - PubMed
-
- Fahy E., Subramaniam S., Brown H. A., Glass C. K., Merrill A. H., Jr, Murphy R. C., Raetz C. R., Russell D. W., Seyama Y., Shaw W., et al. 2005. A comprehensive classification system for lipids. J. Lipid Res. 46: 839–861 - PubMed
-
- Lands W. E., Inoue M., Sugiura Y., Okuyama H. 1982. Selective incorporation of polyunsaturated fatty acids into phosphatidylcholine by rat liver microsomes. J. Biol. Chem. 257: 14968–14972 - PubMed
-
- Diaz B. L., Arm J. P. 2003. Phospholipase A(2). Prostaglandins Leukot. Essent. Fatty Acids. 69: 87–97 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

