Proteolysis controls endogenous substance P levels
- PMID: 23894327
- PMCID: PMC3716696
- DOI: 10.1371/journal.pone.0068638
Proteolysis controls endogenous substance P levels
Abstract
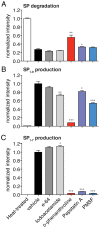
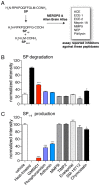
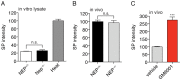
Substance P (SP) is a prototypical neuropeptide with roles in pain and inflammation. Numerous mechanisms regulate endogenous SP levels, including the differential expression of SP mRNA and the controlled secretion of SP from neurons. Proteolysis has long been suspected to regulate extracellular SP concentrations but data in support of this hypothesis is scarce. Here, we provide evidence that proteolysis controls SP levels in the spinal cord. Using peptidomics to detect and quantify endogenous SP fragments, we identify the primary SP cleavage site as the C-terminal side of the ninth residue of SP. If blocking this pathway increases SP levels, then proteolysis controls SP concentration. We performed a targeted chemical screen using spinal cord lysates as a proxy for the endogenous metabolic environment and identified GM6001 (galardin, ilomastat) as a potent inhibitor of the SP(1-9)-producing activity present in the tissue. Administration of GM6001 to mice results in a greater-than-three-fold increase in the spinal cord levels of SP, which validates the hypothesis that proteolysis controls physiological SP levels.
Conflict of interest statement
Figures

References
-
- Ribeiro-da-Silva A, Hokfelt T (2000) Neuroanatomical localisation of substance P in the CNS and sensory neurons. Neuropeptides 34: 256–271. - PubMed
-
- Otsuka M, Yoshioka K (1993) Neurotransmitter functions of mammalian tachykinins. Physiological Reviews 73: 229–308. - PubMed
-
- Hall ME, Stewart JM (1983) Substance P and antinociception. Peptides 4: 31–35. - PubMed
-
- Hall ME, Stewart JM (1983) Substance P and behavior: opposite effects of N-terminal and C-terminal fragments. Peptides 4: 763–768. - PubMed
-
- Yeomans D, Proudfit H (1992) Antinociception induced by microinjection of substance P into the A7 catecholamine cell group in the rat. Neuroscience 49: 681–691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

