Magnetic nanoparticles as mediators of ligand-free activation of EGFR signaling
- PMID: 23894364
- PMCID: PMC3720882
- DOI: 10.1371/journal.pone.0068879
Magnetic nanoparticles as mediators of ligand-free activation of EGFR signaling
Abstract
Background: Magnetic nanoparticles (NPs) are of particular interest in biomedical research, and have been exploited for molecular separation, gene/drug delivery, magnetic resonance imaging, and hyperthermic cancer therapy. In the case of cultured cells, magnetic manipulation of NPs provides the means for studying processes induced by mechanotransduction or by local clustering of targeted macromolecules, e.g. cell surface receptors. The latter are normally activated by binding of their natural ligands mediating key signaling pathways such as those associated with the epidermal growth factor (EGFR). However, it has been reported that EGFR may be dimerized and activated even in the absence of ligands. The present study assessed whether receptor clustering induced by physical means alone suffices for activating EGFR in quiescent cells.
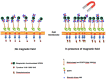
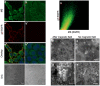
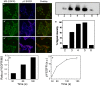
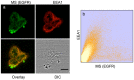
Methodology/principal findings: The EGFR on A431 cells was specifically targeted by superparamagnetic iron oxide NPs (SPIONs) carrying either a ligand-blocking monoclonal anti-EGFR antibody or a streptavidin molecule for targeting a chimeric EGFR incorporating a biotinylated amino-terminal acyl carrier peptide moiety. Application of a magnetic field led to SPION magnetization and clustering, resulting in activation of the EGFR, a process manifested by auto and transphosphorylation and downstream signaling. The magnetically-induced early signaling events were similar to those inherent to the ligand dependent EGFR pathways. Magnetization studies indicated that the NPs exerted magnetic dipolar forces in the sub-piconewton range with clustering dependent on Brownian motion of the receptor-SPION complex and magnetic field strength.
Conclusions/significance: We demonstrate that EGFR on the cell surface that have their ligand binding-pocket blocked by an antibody are still capable of transphosphorylation and initiation of signaling cascades if they are clustered by SPIONs either attached locally or targeted to another site of the receptor ectodomain. The results suggest that activation of growth factor receptors may be triggered by ligand-independent molecular crowding resulting from overexpression and/or sequestration in membrane microdomains.
Conflict of interest statement
Figures

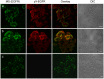
References
-
- Alivisatos AP, Gu W, Larabell C (2005) Quantum Dots as cellular probes. Annu Rev Biomed Eng 7: 55–76. - PubMed
-
- Kim J, Lee JE, Lee SH, Lee JH, Park TG, et al. (2008) Designed fabrication of a multifunctional polymer nanomedical platform for simultaneous cancer- targeted imaging and magnetically guided drug delivery. Adv Mater 20: 478–483.
-
- Jun YW, Seo JW, Cheon J (2008) Nanoscaling Laws of magnetic nanoparticles and their applicabilities in biomedical sciences. Acc Chem Res 41: 179–189. - PubMed
-
- Parak WJ, Pellegrino T, Plank C (2005) Labelling of cells with Quantum Dots. Nanotechnology 16: R9–R25. - PubMed
-
- Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, et al. (2012) Biological applications of magnetic nanoparticles. Chem Soc Rev 41: 4306–4334 10.1039/c2cs15337h. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

