Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction
- PMID: 23894370
- PMCID: PMC3716890
- DOI: 10.1371/journal.pone.0068893
Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction
Abstract
Background: Chronic heart failure (CHF) with preserved left ventricular (LV) ejection fraction (HFpEF) is observed in half of all patients with CHF and carries the same poor prognosis as CHF with reduced LV ejection fraction (HFrEF). In contrast to HFrEF, there is no established therapy for HFpEF. Chronic inflammation contributes to cardiac fibrosis, a crucial factor in HFpEF; however, inflammatory mechanisms and mediators involved in the development of HFpEF remain unclear. Therefore, we sought to identify novel inflammatory mediators involved in this process.
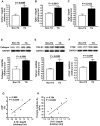
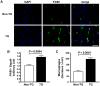
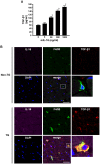
Methods and results: An analysis by multiplex-bead array assay revealed that serum interleukin-16 (IL-16) levels were specifically elevated in patients with HFpEF compared with HFrEF and controls. This was confirmed by enzyme-linked immunosorbent assay in HFpEF patients and controls, and serum IL-16 levels showed a significant association with indices of LV diastolic dysfunction. Serum IL-16 levels were also elevated in a rat model of HFpEF and positively correlated with LV end-diastolic pressure, lung weight and LV myocardial stiffness constant. The cardiac expression of IL-16 was upregulated in the HFpEF rat model. Enhanced cardiac expression of IL-16 in transgenic mice induced cardiac fibrosis and LV myocardial stiffening accompanied by increased macrophage infiltration. Treatment with anti-IL-16 neutralizing antibody ameliorated cardiac fibrosis in the mouse model of angiotensin II-induced hypertension.
Conclusion: Our data indicate that IL-16 is a mediator of LV myocardial fibrosis and stiffening in HFpEF, and that the blockade of IL-16 could be a possible therapeutic option for HFpEF.
Conflict of interest statement
Figures




References
-
- Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, et al. (2004) Trends in heart failure incidence and survival in a community-based population. JAMA 292: 344–350. - PubMed
-
- Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, et al. (2006) Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med 355: 260–269. - PubMed
-
- Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, et al. (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355: 251–259. - PubMed
-
- Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, et al. (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 362: 777–781. - PubMed
-
- Zile MR, Baicu CF, Gaasch WH (2004) Diastolic heart failure–abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 350: 1953–1959. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

