Oxidative burst of circulating neutrophils following traumatic brain injury in human
- PMID: 23894384
- PMCID: PMC3722225
- DOI: 10.1371/journal.pone.0068963
Oxidative burst of circulating neutrophils following traumatic brain injury in human
Erratum in
- PLoS One. 2013;8(9). doi:10.1371/annotation/73ce45d3-4478-4c06-a4d2-3274b6222bae
Abstract
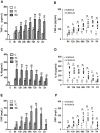
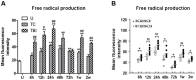
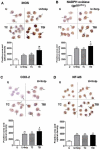
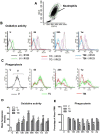
Besides secondary injury at the lesional site, Traumatic brain injury (TBI) can cause a systemic inflammatory response, which may cause damage to initially unaffected organs and potentially further exacerbate the original injury. Here we investigated plasma levels of important inflammatory mediators, oxidative activity of circulating leukocytes, particularly focusing on neutrophils, from TBI subjects and control subjects with general trauma from 6 hours to 2 weeks following injury, comparing with values from uninjured subjects. We observed increased plasma level of inflammatory cytokines/molecules TNF-α, IL-6 and CRP, dramatically increased circulating leukocyte counts and elevated expression of TNF-α and iNOS in circulating leukocytes from TBI patients, which suggests a systemic inflammatory response following TBI. Our data further showed increased free radical production in leukocyte homogenates and elevated expression of key oxidative enzymes iNOS, COX-2 and NADPH oxidase (gp91(phox)) in circulating leukocytes, indicating an intense induction of oxidative burst following TBI, which is significantly greater than that in control subjects with general trauma. Furthermore, flow cytometry assay proved neutrophils as the largest population in circulation after TBI and showed significantly up-regulated oxidative activity and suppressed phagocytosis rate for circulating neutrophils following brain trauma. It suggests that the highly activated neutrophils might play an important role in the secondary damage, even outside the injured brain. Taken together, the potent systemic inflammatory response induced by TBI, especially the intensively increase oxidative activity of circulating leukocytes, mainly neutrophils, may lead to a systemic damage, dysfunction/damage of bystander tissues/organs and even further exacerbate secondary local damage. Controlling these pathophysiological processes may be a promising therapeutic strategy and will protect unaffected organs and the injured brain from the secondary damage.
Conflict of interest statement
Figures

References
-
- Khan F, Baguley IJ, Cameron ID (2003) 4: Rehabilitation after traumatic brain injury. Med J Aust 178: 290–295. - PubMed
-
- Siesjo BK, Siesjo P (1996) Mechanisms of secondary brain injury. Eur J Anaesthesiol 13: 247–268. - PubMed
-
- Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM (1994) Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma 11: 499–506. - PubMed
-
- Baskaran H, Yarmush ML, Berthiaume F (2000) Dynamics of tissue neutrophil sequestration after cutaneous burns in rats. J Surg Res 93: 88–96. - PubMed
-
- Bhatia RK, Pallister I, Dent C, Jones SA, Topley N (2005) Enhanced neutrophil migratory activity following major blunt trauma. Injury 36: 956–962. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

