p16 Stimulates CDC42-dependent migration of hepatocellular carcinoma cells
- PMID: 23894465
- PMCID: PMC3722281
- DOI: 10.1371/journal.pone.0069389
p16 Stimulates CDC42-dependent migration of hepatocellular carcinoma cells
Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide. Tumor dissemination to the extra-hepatic region of the portal vein, lymph nodes, lungs or bones contributes to the high mortality seen in HCC; yet, the molecular mechanisms responsible for HCC metastasis remain unclear. Prior studies have suggested a potential link between accumulated cytoplasm-localized p16 and tumor progression. Here we report that p16 enhances metastasis-associated phenotypes in HCC cells - ectopic p16 expression increased cell migration in vitro, and lung colonization after intravenous injection, whereas knockdown of endogenous p16 reduced cell migration. Interestingly, analysis of p16 mutants indicated that the Cdk4 interaction domain is required for stimulation of HCC cell migration; however, knockdown of Cdk4 and Cdk6 showed that these proteins are dispensable for this phenomenon. Intriguingly, we found that in p16-positive HCC samples, p16 protein is predominantly localized in the cytoplasm. In addition, we identified a potential role for nuclear-cytoplasmic shuttling in p16-stimulated migration, consistent with the predominantly cytoplasmic localization of p16 in IHC-positive HCC samples. Finally, we determined that p16-stimulated cell migration requires the Cdc42 GTPase. Our results demonstrate for the first time a pro-migratory role for p16, and suggest a potential mechanism for the observed association between cytoplasmic p16 and tumor progression in diverse tumor types.
Conflict of interest statement
Figures
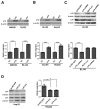

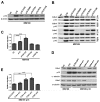

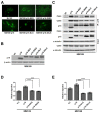
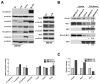
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. doi:10.3322/caac.20107. PubMed: 21296855. - DOI - PubMed
-
- Farazi PA, DePinho RA (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6: 674-687. doi:10.1038/nrc1934. PubMed: 16929323. - DOI - PubMed
-
- Robinson WS (1994) Molecular events in the pathogenesis of hepadnavirus-associated hepatocellular carcinoma. Annu Rev Med 45: 297-323. doi:10.1146/annurev.med.45.1.297. PubMed: 8198385. - DOI - PubMed
-
- Buendia MA (2000) Genetics of hepatocellular carcinoma. Semin Cancer Biol 10: 185-200. doi:10.1006/scbi.2000.0319. PubMed: 10936068. - DOI - PubMed
-
- Liew CT, Li HM, Lo KW, Leow CK, Chan JY et al. (1999) High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene 18: 789-795. doi:10.1038/sj.onc.1202359. PubMed: 9989830. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

