Japanese encephalitis virus disrupts cell-cell junctions and affects the epithelial permeability barrier functions
- PMID: 23894488
- PMCID: PMC3722119
- DOI: 10.1371/journal.pone.0069465
Japanese encephalitis virus disrupts cell-cell junctions and affects the epithelial permeability barrier functions
Abstract
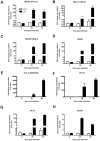
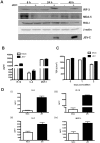
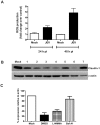
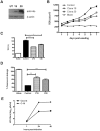
Japanese encephalitis virus (JEV) is a neurotropic flavivirus, which causes viral encephalitis leading to death in about 20-30% of severely-infected people. Although JEV is known to be a neurotropic virus its replication in non-neuronal cells in peripheral tissues is likely to play a key role in viral dissemination and pathogenesis. We have investigated the effect of JEV infection on cellular junctions in a number of non-neuronal cells. We show that JEV affects the permeability barrier functions in polarized epithelial cells at later stages of infection. The levels of some of the tight and adherens junction proteins were reduced in epithelial and endothelial cells and also in hepatocytes. Despite the induction of antiviral response, barrier disruption was not mediated by secreted factors from the infected cells. Localization of tight junction protein claudin-1 was severely perturbed in JEV-infected cells and claudin-1 partially colocalized with JEV in intracellular compartments and targeted for lysosomal degradation. Expression of JEV-capsid alone significantly affected the permeability barrier functions in these cells. Our results suggest that JEV infection modulates cellular junctions in non-neuronal cells and compromises the permeability barrier of epithelial and endothelial cells which may play a role in viral dissemination in peripheral tissues.
Conflict of interest statement
Figures
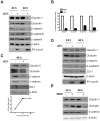
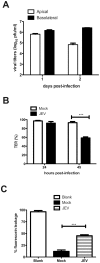
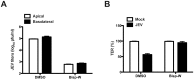
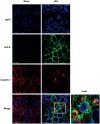
References
-
- Medigeshi GR (2011) Mosquito-borne flaviviruses: overview of viral life-cycle and host‚Äìvirus interactions. Future Virology 6: 1075–1089.
-
- Mishra MK, Dutta K, Saheb SK, Basu A (2009) Understanding the molecular mechanism of blood-brain barrier damage in an experimental model of Japanese encephalitis: correlation with minocycline administration as a therapeutic agent. Neurochem Int 55: 717–723. - PubMed
-
- Swarup V, Ghosh J, Duseja R, Ghosh S, Basu A (2007) Japanese encephalitis virus infection decrease endogenous IL-10 production: correlation with microglial activation and neuronal death. Neurosci Lett 420: 144–149. - PubMed
-
- Huang CH, Wong C (1963) Relation of the peripheral multiplication of Japanese B encephalitis virus to the pathogenesis of the infection in mice. Acta Virol 7: 322–330.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

