CD24 expression identifies teratogen-sensitive fetal neural stem cell subpopulations: evidence from developmental ethanol exposure and orthotopic cell transfer models
- PMID: 23894503
- PMCID: PMC3718834
- DOI: 10.1371/journal.pone.0069560
CD24 expression identifies teratogen-sensitive fetal neural stem cell subpopulations: evidence from developmental ethanol exposure and orthotopic cell transfer models
Abstract
Background: Ethanol is a potent teratogen. Its adverse neural effects are partly mediated by disrupting fetal neurogenesis. The teratogenic process is poorly understood, and vulnerable neurogenic stages have not been identified. Identifying these is a prerequisite for therapeutic interventions to mitigate effects of teratogen exposures.
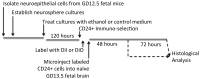
Methods: We used flow cytometry and qRT-PCR to screen fetal mouse-derived neurosphere cultures for ethanol-sensitive neural stem cell (NSC) subpopulations, to study NSC renewal and differentiation. The identity of vulnerable NSC populations was validated in vivo, using a maternal ethanol exposure model. Finally, the effect of ethanol exposure on the ability of vulnerable NSC subpopulations to integrate into the fetal neurogenic environment was assessed following ultrasound guided, adoptive transfer.
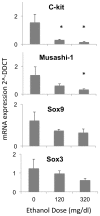
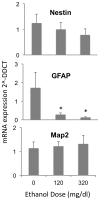
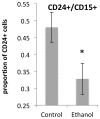
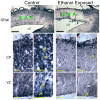
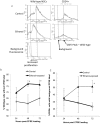
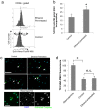
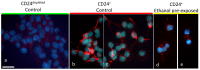
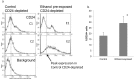
Results: Ethanol decreased NSC mRNAs for c-kit, Musashi-1and GFAP. The CD24(+) NSC population, specifically the CD24(+)CD15(+) double-positive subpopulation, was selectively decreased by ethanol. Maternal ethanol exposure also resulted in decreased fetal forebrain CD24 expression. Ethanol pre-exposed CD24(+) cells exhibited increased proliferation, and deficits in cell-autonomous and cue-directed neuronal differentiation, and following orthotopic transplantation into naïve fetuses, were unable to integrate into neurogenic niches. CD24(depleted) cells retained neurosphere regeneration capacity, but following ethanol exposure, generated increased numbers of CD24(+) cells relative to controls.
Conclusions: Neuronal lineage committed CD24(+) cells exhibit specific vulnerability, and ethanol exposure persistently impairs this population's cell-autonomous differentiation capacity. CD24(+) cells may additionally serve as quorum sensors within neurogenic niches; their loss, leading to compensatory NSC activation, perhaps depleting renewal capacity. These data collectively advance a mechanistic hypothesis for teratogenesis leading to microencephaly.
Conflict of interest statement
Figures



References
-
- Lemoine P, Harouseau H, Borteryu JT, Menuet JC (1968) Les enfants des parents alcooliques: Anomalies observees apropos de 127 cas. Ouest Medical 21: 476–482.
-
- Jones KL, Smith DW, Ulleland CN, Streissguth P (1973) Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1: 1267–1271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

