A simple model system enabling human CD34(+) cells to undertake differentiation towards T cells
- PMID: 23894504
- PMCID: PMC3720953
- DOI: 10.1371/journal.pone.0069572
A simple model system enabling human CD34(+) cells to undertake differentiation towards T cells
Abstract
Background: Channelling the development of haematopoietic progenitor cells into T lymphocytes is dependent upon a series of extrinsic prompts whose temporal and spatial sequence is critical for a productive outcome. Simple models of human progenitor cells development depend in the main on the use of xenogeneic systems which may provide some limitations to development.
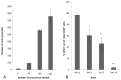
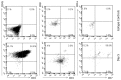
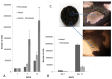
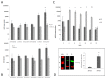
Methods and findings: Here we provide evidence that a simple model system which utilises both human keratinocyte and fibroblast cell lines arrayed on a synthetic tantalum coated matrix provides a permissive environment for the development of human CD34⁺ haematopoietic cells into mature CD4⁺ or CD8⁺ T lymphocytes in the presence of Interleukin 7 (IL-7), Interleukin 15 (IL-15) and the Fms-like tyrosine kinase 3 ligand (Flt-3L). This system was used to compare the ability of CD34(+) cells to produce mature thymocytes and showed that whilst these cells derived from cord blood were able to productively differentiate into thymocytes the system was not permissive for the development of CD34(+) cells from adult peripheral blood.
Conclusions/significance: Our study provides direct evidence for the capacity of human cord blood CD34(+) cells to differentiate along the T lineage in a simple human model system. Productive commitment of the CD34⁺ cells to generate T cells was found to be dependent on a three-dimensional matrix which induced the up-regulation of the Notch delta-like ligand 4 (Dll-4) by epithelial cells.
Conflict of interest statement
Figures



References
-
- De la Hera A, Marston W, Aranda C, Toribio ML, Martinez C (1989) Thymic stroma is required for the development of human T cell lineages in vitro. Int Immunol 1(5): 471-478. doi:10.1093/intimm/1.5.471. PubMed: 2489037. - DOI - PubMed
-
- Mitchell WA, Lang PO, Aspinall R (2010) Tracing thymic output in older individuals. Clin Exp Immunol 161(3): 497-503. doi:10.1111/j.1365-2249.2010.04209.x. PubMed: 20646007. - DOI - PMC - PubMed
-
- Law LW (1966) Restoration of thymic function in neonatally thymectomized mice bearing xenogeneic thymic grafts. Nature 210: 1118. doi:10.1038/2101118a0. PubMed: 5336677. - DOI - PubMed
-
- de Pooter R, Zúñiga-Pflücker JC (2007) T-cell potential and development in vitro: the OP9-DL1 approach. Curr Opin Immunol 19: 163-168. doi:10.1016/j.coi.2007.02.011. PubMed: 17303399. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

