High glucose promotes Aβ production by inhibiting APP degradation
- PMID: 23894546
- PMCID: PMC3720941
- DOI: 10.1371/journal.pone.0069824
High glucose promotes Aβ production by inhibiting APP degradation
Abstract
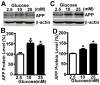
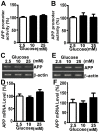
Abnormal deposition of neuriticplaques is the uniqueneuropathological hallmark of Alzheimer's disease (AD).Amyloid β protein (Aβ), the major component of plaques, is generated from sequential cleavage of amyloidβ precursor protein (APP) by β-secretase and γ-secretase complex. Patients with diabetes mellitus (DM), characterized by chronic hyperglycemia,have increased risk of AD development.However, the role of high blood glucose in APP processing and Aβ generation remains elusive. In this study, we investigated the effect of high glucose on APP metabolism and Aβ generation in cultured human cells. We found that high glucose treatment significantly increased APP protein level in both neuronal-like and non-neuronal cells, and promoted Aβ generation. Furthermore, we found that high glucose-induced increase of APP level was not due to enhancement of APP gene transcription but resulted from inhibition of APP protein degradation. Taken together, our data indicated that hyperglycemia could promote AD pathogenesis by inhibiting APP degradation and enhancing Aβ production. More importantly, the elevation of APP level and Aβ generation by high glucose was caused by reduction of APP turnover rate.Thus,our study provides a molecular mechanism of increased risk of developing AD in patients withDMand suggests thatglycemic control might be potentially beneficial for reducing the incidence of AD in diabetic patients and delaying the AD progression.
Conflict of interest statement
Figures

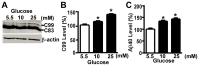
References
-
- Wimo A, Prince M (2010) World Alzheimer Report 2010: The Global Economic Impact of Dementia. Alzheimer’s Disease International, London 1–56.
-
- Querfurth HW, LaFerla FM (2010) Alzheimer’s Disease. New England Journal of Medicine 362: 329–344. - PubMed
-
- Goldgaber D, Lerman MI, McBride OW, Saffiotti U, Gajdusek DC (1987) Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer’s disease. Science 235: 877–880. - PubMed
-
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, et al. (1987) The precursor of Alzheimer’s disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

