Expression of kisspeptins and kiss receptors suggests a large range of functions for kisspeptin systems in the brain of the European sea bass
- PMID: 23894610
- PMCID: PMC3720930
- DOI: 10.1371/journal.pone.0070177
Expression of kisspeptins and kiss receptors suggests a large range of functions for kisspeptin systems in the brain of the European sea bass
Abstract
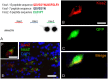
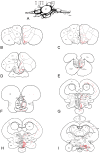
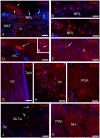
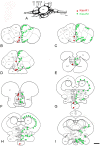
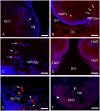
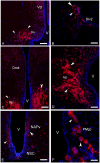
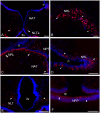
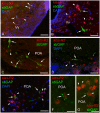
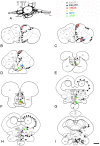
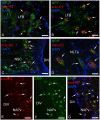
This study, conducted in the brain of a perciform fish, the European sea bass, aimed at raising antibodies against the precursor of the kisspeptins in order to map the kiss systems and to correlate the expression of kisspeptins, kiss1 and kiss2, with that of kisspeptin receptors (kiss-R1 and kiss-R2). Specific antibodies could be raised against the preprokiss2, but not the preoprokiss1. The data indicate that kiss2 neurons are mainly located in the hypothalamus and project widely to the subpallium and pallium, the preoptic region, the thalamus, the pretectal area, the optic tectum, the torus semicircularis, the mediobasal medial and caudal hypothalamus, and the neurohypophysis. These results were compared to the expression of kiss-R1 and kiss-R2 messengers, indicating a very good correlation between the wide distribution of Kiss2-positive fibers and that of kiss-R2 expressing cells. The expression of kiss-R1 messengers was more limited to the habenula, the ventral telencephalon and the proximal pars distalis of the pituitary. Attempts to characterize the phenotype of the numerous cells expressing kiss-R2 showed that neurons expressing tyrosine hydroxylase, neuropeptide Y and neuronal nitric oxide synthase are targets for kisspeptins, while GnRH1 neurons did not appear to express kiss-R1 or kiss-R2 messengers. In addition, a striking result was that all somatostatin-positive neurons expressed-kissR2. These data show that kisspeptins are likely to regulate a wide range of neuronal systems in the brain of teleosts.
Conflict of interest statement
Figures
References
-
- Roa J, Tena-Sempere M (2007) KiSS-1 system and reproduction: comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol 153: 132–140. - PubMed
-
- Tena-Sempere M (2010) Kisspeptins and the metabolic control of reproduction: physiologic roles and physiopathological implications. Ann Endocrinol (Paris) 71: 201–202. - PubMed
-
- Lee YR, Tsunekawa K, Moon MJ, Um HN, Hwang JI, et al. (2009) Molecular evolution of multiple forms of kisspeptins and GPR54 receptors in vertebrates. Endocrinology 150: 2837–2846. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

