Receptor-associated protein blocks internalization and cytotoxicity of myeloma light chain in cultured human proximal tubular cells
- PMID: 23894629
- PMCID: PMC3720907
- DOI: 10.1371/journal.pone.0070276
Receptor-associated protein blocks internalization and cytotoxicity of myeloma light chain in cultured human proximal tubular cells
Abstract
Background: Free light chains (LCs) are among the many ligands that bind to cubilin/megalin for endocytosis via the clathrin-dependent endosomal/lysosomal pathway. Receptor associated protein (RAP), is a 39 kDA high-affinity, chaperone-like ligand for megalin that assists in the proper folding and functioning of megalin/cubilin. Although RAP is known to inhibit ligand binding to megalin/cubilin, its effect on LC endocytosis has not been shown directly.
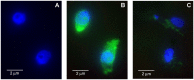
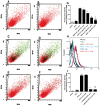
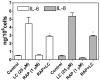
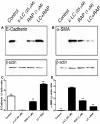
Methods and principal findings: We investigated whether RAP can block the endocytosis of LC in cultured human proximal tubule cells and whether this can prevent LC cytotoxicity. Immunofluorescence microscopy and flow cytometry showed that fluorescently labeled LC endocytosis was markedly inhibited in HK-2 cells pretreated with human RAP. The effect of RAP was dose-dependent, and was predominantly on endocytosis as it had no effect on the small acid-washable fraction of LC bound to cell membrane. RAP significantly inhibited LC induced cytokine production and phosphorylation of ERK1/2 and p38 MAPK. Prolonged exposure to LC for 48 h resulted in epithelial-to-mesenchymal transformation in HK-2 cells as evidenced by marked reduction in the expression of the epithelial cell marker E-cadherin, and increased the expression of the mesenchymal marker α-SMA, which was also prevented by RAP in the endocytosis medium.
Conclusions: RAP inhibited LC endocytosis by ∼88% and ameliorated LC-induced cytokine responses and EMT in human PTCs. The results not only provide additional evidence that LCs endocytosis occurs via the megalin/cubilin endocytic receptor system, but also show that blocking LC endocytosis by RAP can protect proximal tubule cells from LC cytotoxicity.
Conflict of interest statement
Figures

References
-
- Klassen RB, Allen PL, Batuman V, Crenshaw K, Hammond TG (2005) Light chains are a ligand for megalin. Journal of applied physiology 98: 257–263. - PubMed
-
- Batuman V, Verroust PJ, Navar GL, Kaysen JH, Goda FO, et al. (1998) Myeloma light chains are ligands for cubilin (gp280). The American journal of physiology 275: F246–254. - PubMed
-
- Batuman V, Guan S (1997) Receptor-mediated endocytosis of immunoglobulin light chains by renal proximal tubule cells. The American journal of physiology 272: F521–530. - PubMed
-
- Batuman V, Dreisbach AW, Cyran J (1990) Light-chain binding sites on renal brush-border membranes. The American journal of physiology 258: F1259–1265. - PubMed
-
- Nakhoul N, Batuman V (2011) Role of proximal tubules in the pathogenesis of kidney disease. Contributions to nephrology 169: 37–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

