Two modes of cell death caused by exposure to nanosecond pulsed electric field
- PMID: 23894630
- PMCID: PMC3720895
- DOI: 10.1371/journal.pone.0070278
Two modes of cell death caused by exposure to nanosecond pulsed electric field
Abstract
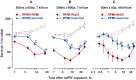
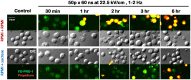
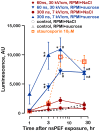
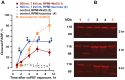
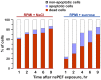
High-amplitude electric pulses of nanosecond duration, also known as nanosecond pulsed electric field (nsPEF), are a novel modality with promising applications for cell stimulation and tissue ablation. However, key mechanisms responsible for the cytotoxicity of nsPEF have not been established. We show that the principal cause of cell death induced by 60- or 300-ns pulses in U937 cells is the loss of the plasma membrane integrity ("nanoelectroporation"), leading to water uptake, cell swelling, and eventual membrane rupture. Most of this early necrotic death occurs within 1-2 hr after nsPEF exposure. The uptake of water is driven by the presence of pore-impermeable solutes inside the cell, and can be counterbalanced by the presence of a pore-impermeable solute such as sucrose in the medium. Sucrose blocks swelling and prevents the early necrotic death; however the long-term cell survival (24 and 48 hr) does not significantly change. Cells protected with sucrose demonstrate higher incidence of the delayed death (6-24 hr post nsPEF). These cells are more often positive for the uptake of an early apoptotic marker dye YO-PRO-1 while remaining impermeable to propidium iodide. Instead of swelling, these cells often develop apoptotic fragmentation of the cytoplasm. Caspase 3/7 activity increases already in 1 hr after nsPEF and poly-ADP ribose polymerase (PARP) cleavage is detected in 2 hr. Staurosporin-treated positive control cells develop these apoptotic signs only in 3 and 4 hr, respectively. We conclude that nsPEF exposure triggers both necrotic and apoptotic pathways. The early necrotic death prevails under standard cell culture conditions, but cells rescued from the necrosis nonetheless die later on by apoptosis. The balance between the two modes of cell death can be controlled by enabling or blocking cell swelling.
Conflict of interest statement
Figures




References
-
- Schoenbach KS, Hargrave B, Joshi RP, Kolb J, Osgood C, et al. (2007) Bioelectric Effects of Nanosecond Pulses. IEEE Transactions on Dielectrics and Electrical Insulation 14: 1088–1109.
-
- Ren W, Sain NM, Beebe SJ (2012) Nanosecond pulsed electric fields (nsPEFs) activate intrinsic caspase-dependent and caspase-independent cell death in Jurkat cells. Biochemical and biophysical research communications 421: 808–812. - PubMed
-
- Beebe SJ, Fox PM, Rec LJ, Somers K, Stark RH, et al. (2002) Nanosecond Pulsed Electric Field (nsPEF) Effects on Cells and Tissues: Apoptosis Induction and Tumor Growth Inhibition. IEEE Transactions on Plasma Science 30: 286–292.
-
- Garon EB, Sawcer D, Vernier PT, Tang T, Sun Y, et al. (2007) In vitro and in vivo evaluation and a case report of intense nanosecond pulsed electric field as a local therapy for human malignancies. Int J Cancer 121: 675–682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

