Lithium induces ER stress and N-glycan modification in galactose-grown Jurkat cells
- PMID: 23894652
- PMCID: PMC3718757
- DOI: 10.1371/journal.pone.0070410
Lithium induces ER stress and N-glycan modification in galactose-grown Jurkat cells
Abstract
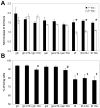
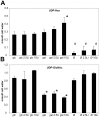
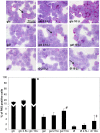
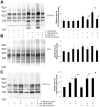
We previously reported that lithium had a significant impact on Ca(2+) regulation and induced unfolded protein response (UPR) in yeast cells grown on galactose due to inhibition of phosphoglucomutase (PGM), however the exact mechanism has not been established yet. In this study, we analysed lithium's effect in galactose-fed cells to clarify whether these ER-related changes are the result of a relative hypoglycemic state. Furthermore, we investigated whether the alterations in galactose metabolism impact protein post-translational modifications. Thus, Jurkat cells were incubated in glucose or galactose containing media with or without lithium treatment. We found that galactose-fed and lithium treated cells showed better survivability than fasting cells. We also found higher UDP-Hexose and glycogen levels in these cells compared to fasting cells. On the other hand, the UPR (X-box binding protein 1 mRNA levels) of galactose-fed and lithium treated cells was even greater than in fasting cells. We also found increased amount of proteins that contained N-linked N-acetyl-glucosamine, similar to what was reported in fasting cells by a recent study. Our results demonstrate that lithium treatment of galactose-fed cells can induce stress responses similar to hypoglycemia, however cell survival is still secured by alternative pathways. We propose that clarifying this process might be an important addition toward the better understanding of the molecular mechanisms that regulate ER-associated stress response.
Conflict of interest statement
Figures




Similar articles
-
The yeast protein Ubx4p contributes to mitochondrial respiration and lithium-galactose-mediated activation of the unfolded protein response.J Biol Chem. 2020 Mar 20;295(12):3773-3782. doi: 10.1074/jbc.RA119.011271. Epub 2020 Jan 29. J Biol Chem. 2020. PMID: 31996377 Free PMC article.
-
Phosphoglucomutase is an in vivo lithium target in yeast.J Biol Chem. 2001 Oct 12;276(41):37794-801. doi: 10.1074/jbc.M101451200. Epub 2001 Aug 10. J Biol Chem. 2001. PMID: 11500487
-
Polychlorinated biphenyl quinone induces endoplasmic reticulum stress, unfolded protein response, and calcium release.Chem Res Toxicol. 2015 Jun 15;28(6):1326-37. doi: 10.1021/acs.chemrestox.5b00124. Epub 2015 May 18. Chem Res Toxicol. 2015. PMID: 25950987
-
An Overview of Unfolded Protein Response Signaling and Its Role in Cancer.Cancer Biother Radiopharm. 2017 Oct;32(8):275-281. doi: 10.1089/cbr.2017.2309. Cancer Biother Radiopharm. 2017. PMID: 29053418 Review.
-
Systemic effects of AGEs in ER stress induction in vivo.Glycoconj J. 2016 Aug;33(4):537-44. doi: 10.1007/s10719-016-9680-4. Epub 2016 May 28. Glycoconj J. 2016. PMID: 27236787 Review.
Cited by
-
4-Phenylbutyric Acid (4-PBA) and Lithium Cooperatively Attenuate Cell Death during Oxygen-Glucose Deprivation (OGD) and Reoxygenation.Cell Mol Neurobiol. 2015 Aug;35(6):849-59. doi: 10.1007/s10571-015-0179-5. Epub 2015 Mar 17. Cell Mol Neurobiol. 2015. PMID: 25776137 Free PMC article.
-
The unfolded protein response has a protective role in yeast models of classic galactosemia.Dis Model Mech. 2014 Jan;7(1):55-61. doi: 10.1242/dmm.012641. Epub 2013 Sep 25. Dis Model Mech. 2014. PMID: 24077966 Free PMC article.
-
A Genome-Wide Screen in Saccharomyces cerevisiae Reveals a Critical Role for Oxidative Phosphorylation in Cellular Tolerance to Lithium Hexafluorophosphate.Cells. 2021 Apr 13;10(4):888. doi: 10.3390/cells10040888. Cells. 2021. PMID: 33924665 Free PMC article.
-
The yeast protein Ubx4p contributes to mitochondrial respiration and lithium-galactose-mediated activation of the unfolded protein response.J Biol Chem. 2020 Mar 20;295(12):3773-3782. doi: 10.1074/jbc.RA119.011271. Epub 2020 Jan 29. J Biol Chem. 2020. PMID: 31996377 Free PMC article.
References
-
- Acosta PB, Gross KC (1995) Hidden sources of galactose in the environment. Eur J Pediatr 154: : S87–92. Available:http://www.ncbi.nlm.nih.gov/pubmed/7671974. Accessed 21 March 2012. - PubMed
-
- Vannucci SJ, Maher F, Simpson I a (1997) Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia 21: : 2–21. Available:http://www.ncbi.nlm.nih.gov/pubmed/9298843. Accessed 1 August 2011. - PubMed
-
- Berry GT, Nissim I, Lin Z, Mazur AT, Gibson JB, et al.. (1995) Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet 346: : 1073–1074. Available:http://www.ncbi.nlm.nih.gov/pubmed/7564790. Accessed 21 March 2012. - PubMed
-
- Lorenzi M (2007) The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res 2007: 61038. Available:http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1950230&tool=p.... Accessed 6 March 2012. - PMC - PubMed
-
- Masuda CA, Xavier MA, Mattos KA, Galina A, Montero-Lomeli M (2001) Phosphoglucomutase is an in vivo lithium target in yeast. J Biol Chem 276: : 37794–37801. Available:http://www.ncbi.nlm.nih.gov/pubmed/11500487. Accessed 21 March 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

