Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression
- PMID: 23894654
- PMCID: PMC3722166
- DOI: 10.1371/journal.pone.0070417
Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression
Abstract
Purpose: Radiofrequency ablation (RFA) is a minimally invasive energy delivery technique increasingly used for focal therapy to eradicate localized disease. RFA-induced tumor-cell necrosis generates an immunogenic source of tumor antigens known to induce antitumor immune responses. However, RFA-induced antitumor immunity is insufficient to control metastatic progression. We sought to characterize (a) the role of RFA dose on immunogenic modulation of tumor and generation of immune responses and (b) the potential synergy between vaccine immunotherapy and RFA aimed at local tumor control and decreased systemic progression.
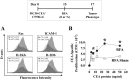
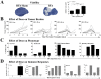
Experimental design: Murine colon carcinoma cells expressing the tumor-associated (TAA) carcinoembryonic antigen (CEA) (MC38-CEA(+)) were studied to examine the effect of sublethal hyperthermia in vitro on the cells' phenotype and sensitivity to CTL-mediated killing. The effect of RFA dose was investigated in vivo impacting (a) the phenotype and growth of MC38-CEA(+) tumors and (b) the induction of tumor-specific immune responses. Finally, the molecular signature was evaluated as well as the potential synergy between RFA and poxviral vaccines expressing CEA and a TRIad of COstimulatory Molecules (CEA/TRICOM).
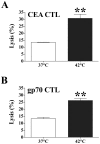
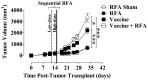
Results: In vitro, sublethal hyperthermia of MC38-CEA(+) cells (a) increased cell-surface expression of CEA, Fas, and MHC class I molecules and (b) rendered tumor cells more susceptible to CTL-mediated lysis. In vivo, RFA induced (a) immunogenic modulation on the surface of tumor cells and (b) increased T-cell responses to CEA and additional TAAs. Combination therapy with RFA and vaccine in CEA-transgenic mice induced a synergistic increase in CD4(+) T-cell immune responses to CEA and eradicated both primary CEA(+) and distal CEA(-) s.c. tumors. Sequential administration of low-dose and high-dose RFA with vaccine decreased tumor recurrence compared to RFA alone. These studies suggest a potential clinical benefit in combining RFA with vaccine in cancer patients, and augment support for this novel translational paradigm.
Conflict of interest statement
Figures


References
-
- Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, et al. (2011) Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 13: e252–265. - PubMed
-
- Gervais DA, Goldberg SN, Brown DB, Soulen MC, Millward SF, et al. (2009) Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 20: S342–347. - PubMed
-
- Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, et al. (2006) Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res 66: 1139–1146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

