Binding of the biogenic polyamines to deoxyribonucleic acids of varying base composition: base specificity and the associated energetics of the interaction
- PMID: 23894663
- PMCID: PMC3722294
- DOI: 10.1371/journal.pone.0070510
Binding of the biogenic polyamines to deoxyribonucleic acids of varying base composition: base specificity and the associated energetics of the interaction
Abstract
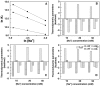
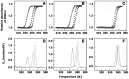
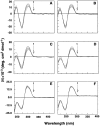
Background: The thermodynamics of the base pair specificity of the binding of the polyamines spermine, spermidine, putrescine, and cadaverine with three genomic DNAs Clostridium perfringens, 27% GC, Escherichia coli, 50% GC and Micrococcus lysodeikticus, 72% GC have been studied using titration calorimetry and the data supplemented with melting studies, ethidium displacement and circular dichroism spectroscopy results.
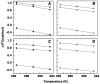
Methodology/principal findings: Isothermal titration calorimetry, differential scanning calorimetry, optical melting studies, ethidium displacement, circular dichroism spectroscopy are the various techniques employed to characterize the interaction of four polyamines, spermine, spermidine, putersine and cadaverine with the DNAs. Polyamines bound stronger with AT rich DNA compared to the GC rich DNA and the binding varied depending on the charge on the polyamine as spermine>spermidine >putrescine>cadaverine. Thermodynamics of the interaction revealed that the binding was entropy driven with small enthalpy contribution. The binding was influenced by salt concentration suggesting the contribution from electrostatic forces to the Gibbs energy of binding to be the dominant contributor. Each system studied exhibited enthalpy-entropy compensation. The negative heat capacity changes suggested a role for hydrophobic interactions which may arise due to the non polar interactions between DNA and polyamines.
Conclusion/significance: From a thermodynamic analysis, the AT base specificity of polyamines to DNAs has been elucidated for the first time and supplemented by structural studies.
Conflict of interest statement
Figures



References
-
- Heby O (1981) Role of polyamines in the control of cell proliferation and differentiation. Differentiation 19: 1–20. - PubMed
-
- Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem 53: 749–790. - PubMed
-
- Thomas TJ, Bloomfield VA (1985) Quasielastic laser light scattering and electron microscopy studies of the conformational transitions and condensation of poly(dA-dT) · poly(dA-dT). Biopolymers 12: 2185–2194. - PubMed
-
- Pegg AE (1988) Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res 48: 759–774. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

