Refolding of a thermostable glyceraldehyde dehydrogenase for application in synthetic cascade biomanufacturing
- PMID: 23894676
- PMCID: PMC3722153
- DOI: 10.1371/journal.pone.0070592
Refolding of a thermostable glyceraldehyde dehydrogenase for application in synthetic cascade biomanufacturing
Abstract
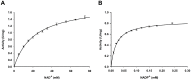
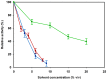
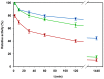
The production of chemicals from renewable resources is gaining importance in the light of limited fossil resources. One promising alternative to widespread fermentation based methods used here is Synthetic Cascade Biomanufacturing, the application of minimized biocatalytic reaction cascades in cell free processes. One recent example is the development of the phosphorylation independent conversion of glucose to ethanol and isobutanol using only 6 and 8 enzymes, respectively. A key enzyme for this pathway is aldehyde dehydrogenase from Thermoplasma acidophilum, which catalyzes the highly substrate specific oxidation of d-glyceraldehyde to d-glycerate. In this work the enzyme was recombinantly expressed in Escherichia coli. Using matrix-assisted refolding of inclusion bodies the yield of enzyme production was enhanced 43-fold and thus for the first time the enzyme was provided in substantial amounts. Characterization of structural stability verified correct refolding of the protein. The stability of the enzyme was determined by guanidinium chloride as well as isobutanol induced denaturation to be ca. -8 kJ/mol both at 25°C and 40°C. The aldehyde dehydrogenase is active at high temperatures and in the presence of small amounts of organic solvents. In contrast to previous publications, the enzyme was found to accept NAD(+) as cofactor making it suitable for application in the artificial glycolysis.
Conflict of interest statement
Figures


Similar articles
-
Improvement of thermostable aldehyde dehydrogenase by directed evolution for application in Synthetic Cascade Biomanufacturing.Enzyme Microb Technol. 2013 Oct 10;53(5):307-14. doi: 10.1016/j.enzmictec.2013.07.002. Epub 2013 Jul 17. Enzyme Microb Technol. 2013. PMID: 24034429
-
Identification and characterization of Thermoplasma acidophilum glyceraldehyde dehydrogenase: a new class of NADP+-specific aldehyde dehydrogenase.Biochem J. 2006 Jul 1;397(1):131-8. doi: 10.1042/BJ20051763. Biochem J. 2006. PMID: 16566751 Free PMC article.
-
Molecular Dynamics Analysis of a Rationally Designed Aldehyde Dehydrogenase Gives Insights into Improved Activity for the Non-Native Cofactor NAD.ACS Synth Biol. 2020 Apr 17;9(4):920-929. doi: 10.1021/acssynbio.9b00527. Epub 2020 Apr 7. ACS Synth Biol. 2020. PMID: 32208678
-
Cell-free biosystems for biomanufacturing.Adv Biochem Eng Biotechnol. 2013;131:89-119. doi: 10.1007/10_2012_159. Adv Biochem Eng Biotechnol. 2013. PMID: 23111502 Review.
-
Metabolic engineering of Escherichia coli for the production of isobutanol: a review.World J Microbiol Biotechnol. 2021 Sep 6;37(10):168. doi: 10.1007/s11274-021-03140-0. World J Microbiol Biotechnol. 2021. PMID: 34487256 Review.
Cited by
-
Cell-free metabolic engineering: biomanufacturing beyond the cell.Biotechnol J. 2015 Jan;10(1):69-82. doi: 10.1002/biot.201400330. Epub 2014 Oct 15. Biotechnol J. 2015. PMID: 25319678 Free PMC article. Review.
-
Advanced Insights into Catalytic and Structural Features of the Zinc-Dependent Alcohol Dehydrogenase from Thauera aromatica.Chembiochem. 2022 Aug 3;23(15):e202200149. doi: 10.1002/cbic.202200149. Epub 2022 Jun 14. Chembiochem. 2022. PMID: 35557486 Free PMC article.
-
Crystallization behaviour of glyceraldehyde dehydrogenase from Thermoplasma acidophilum.Acta Crystallogr F Struct Biol Commun. 2015 Dec;71(Pt 12):1475-80. doi: 10.1107/S2053230X15020270. Epub 2015 Nov 18. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26625289 Free PMC article.
-
Optimization of a reduced enzymatic reaction cascade for the production of L-alanine.Sci Rep. 2019 Aug 13;9(1):11754. doi: 10.1038/s41598-019-48151-y. Sci Rep. 2019. PMID: 31409820 Free PMC article.
-
An NAD-Specific 6-Hydroxy-3-Succinoyl-Semialdehyde-Pyridine Dehydrogenase from Nicotine-Degrading Agrobacterium tumefaciens Strain S33.Microbiol Spectr. 2021 Sep 3;9(1):e0092421. doi: 10.1128/Spectrum.00924-21. Epub 2021 Aug 11. Microbiol Spectr. 2021. PMID: 34378958 Free PMC article.
References
-
- Guterl J-K, Sieber V (2013) Biosynthesis “debugged”: Novel bioproduction strategies. Engineering in Life Sciences 13: 4–18.
-
- Algar EM, Scopes RK (1985) Studies on cell-free metabolism - Ethanol-production by extracts of Zymomonas mobilis . Journal of Biotechnology 2: 275–287.
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

