Hydrogels Formed by Oxo-ester Mediated Native Chemical Ligation
- PMID: 23894696
- PMCID: PMC3719992
- DOI: 10.1039/C3BM00201B
Hydrogels Formed by Oxo-ester Mediated Native Chemical Ligation
Abstract
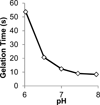
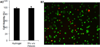
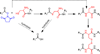
Oxo-ester mediated native chemical ligation (OMNCL) is a variation of the more general native chemical ligation (NCL) reaction that is widely employed for chemoselective ligation of peptide fragments. While OMNCL has been used for a variety of peptide ligations and for biomolecular modification of surfaces, it is typically practiced under harsh conditions that are unsuitable for use in a biological context. In this report we describe the use of OMNCL for polymer hydrogel formation, in-vitro cell encapsulation, and in-vivo implantation. Multivalent polymer precursors containing N-hydroxysuccinimide (NHS) activated oxo-esters and N-cysteine (N-Cys) endgroups were chemically synthesized from branched poly(ethylene glycol) (PEG). Hydrogels formed rapidly at physiologic pH upon mixing of aqueous solutions of NHS and N-Cys functionalized PEGs. Quantitative 1H NMR experiments showed that the reaction proceeds through an OMNCL pathway involving thiol capture to form a thioester intermediate, followed by an S-to-N acyl rearrangement to yield an amide cross-link. pH and temperature were found to influence gelation rate, allowing tailoring of gelation times from a few seconds to a few minutes. OMNCL hydrogels initially swelled before contracting to reach an equilibrium increase in relative wet weight of 0%. This unique behavior impacted the gel stiffness and was attributed to latent formation of disulfide cross-links between network-bound Cys residues. OMNCL hydrogels were adhesive to hydrated tissue, generating a lap shear adhesion strength of 46 kPa. Cells encapsulated in OMNCL hydrogels maintained high viability, and in-situ formation of OMNCL hydrogel by subcutaneous injection in mice generated a minimal acute inflammatory response. OMNCL represents a promising strategy for chemical cross-linking of hydrogels in a biological context and is an attractive candidate for in-vivo applications such as wound healing, tissue repair, drug delivery, and tissue engineering.
Figures

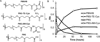



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

