C-fos down-regulation inhibits testosterone-dependent male sexual behavior and the associated learning
- PMID: 23895306
- PMCID: PMC3818384
- DOI: 10.1111/ejn.12321
C-fos down-regulation inhibits testosterone-dependent male sexual behavior and the associated learning
Abstract
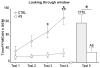
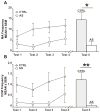
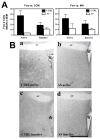
Environmental stimulation results in an increased expression of transcription factors called immediate early genes (IEGs) in specific neuronal populations. In male Japanese quail, copulation with a female increases the expression of the IEGs zenk and c-fos in the medial pre-optic nucleus (POM), a key nucleus controlling male sexual behavior. The functional significance of this increased IEG expression that follows performance of copulatory behavior is unknown. We addressed this question by repeatedly quantifying the performance of appetitive (learned social proximity response) and consummatory (actual copulation) sexual behavior in castrated, testosterone-treated males that received daily intra-cerebroventricular injection of an antisense oligodeoxynucleotide targeting c-fos or control vehicle. Daily antisense injections significantly inhibited the expression of copulatory behavior as well as the acquisition of the learned social proximity response. A strong reduction of the proximity response was still observed in antisense-treated birds that copulated with a female, ruling out the indirect effect of the absence of interactions with females on the learning process. After a 2-day interruption of behavioral testing but not of antisense injections, birds were submitted to a final copulatory test that confirmed the behavioral inhibition in antisense-injected birds. Brains were collected at 90 min after the behavioral testing for quantification of c-fos-immunoreactive cells. A significant reduction of the number of c-fos-positive cells in the POM but not in other brain regions was observed following antisense injection. Taken together, the data suggest that c-fos expression in the POM modulates copulatory behavior and sexual learning in male quail.
Keywords: Japanese quail; appetitive sexual behavior; c-fos antisense; medial pre-optic nucleus; sexual learning.
© 2013 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures

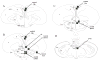


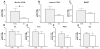
Similar articles
-
Fos induction in the Japanese quail brain after expression of appetitive and consummatory aspects of male sexual behavior.Brain Res Bull. 2000 Jul 1;52(4):249-62. doi: 10.1016/s0361-9230(00)00233-1. Brain Res Bull. 2000. PMID: 10856822
-
Performance of appetitive or consummatory components of male sexual behavior is mediated by different brain areas: a 2-deoxyglucose autoradiographic study.Neuroscience. 1999;94(4):1261-77. doi: 10.1016/s0306-4522(99)00318-8. Neuroscience. 1999. PMID: 10625066
-
Medial Preoptic Regulation of the Ventral Tegmental Area Related to the Control of Sociosexual Behaviors.eNeuro. 2017 Jan 9;3(6):ENEURO.0283-16.2016. doi: 10.1523/ENEURO.0283-16.2016. eCollection 2016 Nov-Dec. eNeuro. 2017. PMID: 28083561 Free PMC article.
-
The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior.Front Neuroendocrinol. 1996 Jan;17(1):51-125. doi: 10.1006/frne.1996.0002. Front Neuroendocrinol. 1996. PMID: 8788569 Review.
-
Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors.Front Neuroendocrinol. 2007 Oct;28(4):161-78. doi: 10.1016/j.yfrne.2007.05.003. Epub 2007 Jun 8. Front Neuroendocrinol. 2007. PMID: 17624413 Free PMC article. Review.
Cited by
-
Rapid changes in brain estrogen concentration during male sexual behavior are site and stimulus specific.Sci Rep. 2021 Oct 11;11(1):20130. doi: 10.1038/s41598-021-99497-1. Sci Rep. 2021. PMID: 34635715 Free PMC article.
-
Expression and Functional Characterization of c-Fos Gene in Chinese Fire-Bellied Newt Cynops Orientalis.Genes (Basel). 2021 Jan 30;12(2):205. doi: 10.3390/genes12020205. Genes (Basel). 2021. PMID: 33573315 Free PMC article.
-
c-Fos downregulation positively regulates EphA5 expression in a congenital hypothyroidism rat model.J Mol Histol. 2018 Apr;49(2):147-155. doi: 10.1007/s10735-018-9754-7. Epub 2018 Jan 12. J Mol Histol. 2018. PMID: 29330744
-
Differential control of appetitive and consummatory sexual behavior by neuroestrogens in male quail.Horm Behav. 2018 Aug;104:15-31. doi: 10.1016/j.yhbeh.2018.02.006. Epub 2018 Feb 21. Horm Behav. 2018. PMID: 29452074 Free PMC article. Review.
References
-
- Abraham WC, Dragunow M, Tate WP. The role of immediate early genes in the stabilization of long-term potentiation. Molecular neurobiology. 1991;5:297–314. - PubMed
-
- Adkins EK, Adler NT. Hormonal control of behavior in the Japanese quail. J Comp Physiol Psychol. 1972;81:27–36. - PubMed
-
- Aste N, Panzica GC, Viglietti-Panzica C, Harada N, Balthazart J. Distribution and effects of testosterone on aromatase mRNA in the quail forebrain: A non-radioactive in situ hybridization study. J Chem Neuroanat. 1998;14:103–115. - PubMed
-
- Aste N, Viglietti-Panzica C, Balthazart J, Panzica GC. Testosterone modulation of peptidergic pathways in the septo-preoptic region of male Japanese quail. Poultry and Avian Biology Reviews. 1997;8:77–93.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

