Evaluation of the effect of cobicistat on the in vitro renal transport and cytotoxicity potential of tenofovir
- PMID: 23896476
- PMCID: PMC3811427
- DOI: 10.1128/AAC.00712-13
Evaluation of the effect of cobicistat on the in vitro renal transport and cytotoxicity potential of tenofovir
Abstract
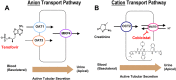
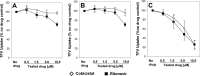
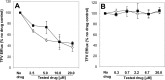
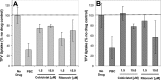
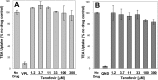
A once-daily single-tablet antiretroviral regimen containing tenofovir (TFV) disoproxil fumarate, emtricitabine (FTC), elvitegravir (EVG), and cobicistat (COBI) is an approved combination for the treatment of patients infected with HIV. COBI and TFV have been reported to interact with distinct transporters in renal proximal tubules; while TFV is renally eliminated by a combination of glomerular filtration and tubular secretion via anion transporters OAT1, OAT3, and MRP4, COBI inhibits renal cation transporters, particularly MATE1, resulting in a measurable decrease in the tubular secretion of creatinine. To investigate the potential for a renal drug-drug interaction between TFV and COBI in vitro, the uptake of TFV in the presence and absence of COBI was determined in fresh human renal cortex tissue and in cells expressing the relevant renal transporters. At concentrations exceeding clinical protein-unbound plasma levels, COBI did not significantly inhibit the transport of TFV by the anion transporters OAT1, OAT3, and MRP4 (50% inhibitory concentrations [IC50s] of >15, 6.6, and 8.5 μM, respectively). Conversely, TFV had little or no effect on the cation transporters OCT2 and MATE1 (IC50 > 100 μM). Consistent with studies using individual transporters, no increase in the accumulation of TFV in freshly isolated human renal cortex tissue or renal proximal tubule cells (RPTECs) was observed in the presence of COBI. Finally, COBI alone or in combination with FTC and EVG did not affect the sensitivity to TFV of cultured primary RPTECs or cells coexpressing OAT1 and MRP4. These results illustrate that COBI and TFV interact primarily with distinct renal transporters and indicate a low potential for pharmacokinetic renal drug-drug interaction.
Figures
References
-
- Mathias AA, German P, Murray BP, Wei L, Jain A, West S, Warren D, Hui J, Kearney BP. 2010. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin. Pharmacol. Ther. 87:322–329 - PubMed
-
- Ramanathan S, Mathias AA, German P, Kearney BP. 2011. Clinical pharmacokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin. Pharmacokinet. 50:229–244 - PubMed
-
- Gallant J, Koenig E, Andrade-Villanueva J, Chetchotisakd P, DeJesus E, Antunes F, Arastéh K, Moyle G, Rizzardini G, Fehr J, Liu Y, Zhong L, Callebaut C, Ramanathan S, Szwarcberg J, Rhee M, Cheng A. 2012. Cobicistat versus ritonavir as pharmacoenhancers in combination with atazanavir plus tenofovirDF/emtricitabine: phase 3 randomized, double blind, active-controlled trial, week 48 results. J. Int. AIDS Soc. 15(Suppl 3):53
-
- Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, Gallant JE, Liu HC, Zhong L, Yale K, White K, Kearney BP, Szwarcberg J, Quirk E, Cheng AK. 2012. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet 379:2439–2448 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

