Safety, tolerability, pharmacokinetics, and antiviral activity of GSK2336805, an inhibitor of hepatitis C virus (HCV) NS5A, in healthy subjects and subjects chronically infected with HCV genotype 1
- PMID: 23896477
- PMCID: PMC3811459
- DOI: 10.1128/AAC.00910-13
Safety, tolerability, pharmacokinetics, and antiviral activity of GSK2336805, an inhibitor of hepatitis C virus (HCV) NS5A, in healthy subjects and subjects chronically infected with HCV genotype 1
Abstract
GSK2336805 is an orally bioavailable hepatitis C virus (HCV) inhibitor working through an NS5A-mediated mechanism. This first-time-in-human study was conducted to assess the safety, tolerability, pharmacokinetics, metabolism, and efficacy of GSK2336805 in healthy subjects and subjects infected with HCV genotype 1. We performed a three-part, randomized, double-blind, placebo-controlled study in 46 healthy subjects and 23 HCV-infected subjects. After an overnight fast, healthy subjects received GSK2336805 as 10 mg, 30 mg, 30 mg plus food, and 60 mg in a single dose and 10 mg (7 days), 30 mg (7 days), and 75 mg (14 days) in a once-daily multiple dose. Subjects with HCV received GSK2336805 as a 1- to 120-mg single dose. In subjects with HCV, reductions in HCV RNA were observed within 4 h and a single dose of GSK2336805 of ≥10 mg resulted in a statistically significant ≥2-log reduction in HCV RNA compared with placebo at 24 h postdose. GSK2336805 was readily absorbed in all subjects, and the half-life (t1/2) was suitable for once-daily dosing. Administration of GSK2336805 with food had no effect on plasma GSK2336805 exposure; however, absorption was delayed, with a median tmax (time to maximum concentration of drug in serum) of 4.5 versus 2.0 h. Twenty subjects who received GSK2336805 experienced mild to moderate adverse events; none were serious. GSK2336805 was well tolerated and exhibited rapid, significant antiviral activity after a single dose in HCV-infected subjects. These results support the conduct of further studies evaluating GSK2336805 administered once daily for longer durations in combination with peginterferon, ribavirin, and other direct-acting antivirals. (This study has been registered at ClinicalTrials.gov under registration no. NCT01277692.).
Figures

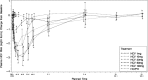

References
-
- Lauer GM, Walker BD. 2001. Hepatitis C virus infection. N. Engl. J. Med. 345:41–52 - PubMed
-
- Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H, Elshazly M, Esmat G, Guan R, Han KH, Koike K, Largen A, McCaughan G, Mogawer S, Monis A, Nawaz A, Piratvisuth T, Sanai FM, Sharara AI, Sibbel S, Sood A, Suh DJ, Wallace C, Young K, Negro F. 2011. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 31(Suppl 2):61–80 - PubMed
-
- Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, Dalgard O, Dillion JF, Flisiak R, Forns X, Frankova S, Goldis A, Goulis I, Halota W, Hunyady B, Lagging M, Largen A, Makara M, Manolakopoulos S, Marcellin P, Marinho RT, Pol S, Poynard T, Puoti M, Sagalova O, Sibbel S, Simon K, Wallace C, Young K, Yurdaydin C, Zuckerman E, Negro F, Zeuzem S. 2011. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 31(Suppl 2):30–60 - PubMed
-
- Kershenobich D, Razavi HA, Sánchez-Avila JF, Bessone F, Coelho HS, Dagher L, Gonçales FL, Quiroz JF, Rodriguez-Perez F, Rosado B, Wallace C, Negro F, Silva M. 2011. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int. 31(Suppl 2):18–29 - PubMed
-
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S, Study Team ADVANCE 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364:2405–2416 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

