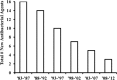
The FDA reboot of antibiotic development
- PMID: 23896479
- PMCID: PMC3811409
- DOI: 10.1128/AAC.01277-13
The FDA reboot of antibiotic development
Figures
References
-
- Spellberg B, Brass EP, Bradley JS, Lewis R, Shlaes D, Ambrose P, Das A, Boucher HW, Doi Y, Bartlett JG, Bonomo RA, Larosa SP, Talbot GH, Benjamin D, Guidos RJ, Jezek A, Gilbert DN, on behalf of the Infectious Diseases Society of America 2012. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin. Infect. Dis. 55:1031–1046 - PMC - PubMed
-
- Rex JH, Eisenstein BI, Alder J, Goldberger M, Meyer R, Dane A, Friedland I, Knirsch C, Sanhai WR, Tomayko J, Lancaster C, Jackson J. 2013. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect. Dis. 13:269–275 - PubMed
-
- Shlaes DM, Moellering RC., Jr 2002. The United States Food and Drug Administration and the end of antibiotics. Clin. Infect. Dis. 34:420–422 - PubMed
-
- Projan SJ, Shlaes DM. 2004. Antibacterial drug discovery: is it all downhill from here? Clin. Microbiol. Infect. 10(Suppl 4):18–22 - PubMed
-
- Sharma P, Towse A. 2011. New drugs to tackle antimicrobial resistance: analysis of EU policy options. Office of Health Economics, London, United Kingdom: http://www.ohe.org/publications/article/new-drugs-to-tackle-antimicrobia...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical