Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults
- PMID: 23896606
- PMCID: PMC3906363
- DOI: 10.4161/hv.25458
Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults
Abstract
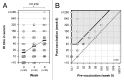
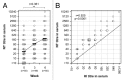
Haemagglutination inhibition (HI) and neutralization (NT) titers as well as haemagglutinin (HA) specific antibody responses were examined in 50 healthy adults aged between 22 and 69 y old after two intranasal administrations of an inactivated whole virus vaccine derived from A/Victoria/210/2009 virus (45 μg HA per dose) at 3 week intervals. Serum HI titers after two-doses of the nasal vaccine showed>2.5-fold rise in the ratio of geometric mean titer upon vaccination,>40% of subjects with a ≥ 4-fold increase in titer and>70% of subjects with a titer of ≥ 1:40, all parameters associated with an effective outcome of vaccination in the criteria defined by the European Medicines Agency. Serum neutralizing antibody responses correlated with HI antibody responses, although NT titers were about 2-fold higher than HI titers. These high levels of serum responses were accompanied by high levels of HI and neutralizing antibody responses in nasal mucus as measured in concentrated nasal wash samples that were about 10 times diluted compared with natural nasal mucus. Serum and nasal HI and neutralizing antibody responses consisted of HA-specific IgG and IgA antibody responses, with IgG and IgA antibodies being dominant in serum and nasal responses, respectively.
Keywords: haemagglutination-inhibiting antibody; healthy adult volunteer; influenza virus; intranasal vaccination; neutralizing antibody.
Figures

References
-
- Murphy BR, Webster RG. Orthomyxoviruses. In: Fields BN, Knipe DM, M. HP, Chanock RM, Melnick JL, Monath TP, et al., eds. Fields Virology. Philadelphia: Lippincott Williams & Wilkins, 1996:1397-445.
-
- Murphy BR. Mucosal Immunity to Viruses. In: Ogra PL, Mestecky J, Lamm ME, Strober W, McGee JR, Bienenstock J, eds. Handbook of Mucosal Immunology. San Diego: Academic Press, 1994:333-43.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
