Effect of ring-constrained phenylpropyloxyethylamines on sigma receptors
- PMID: 23896610
- PMCID: PMC8463110
- DOI: 10.1016/j.bmc.2013.06.068
Effect of ring-constrained phenylpropyloxyethylamines on sigma receptors
Abstract
A series of ring-constrained phenylpropyloxyethylamines, partial opioid structure analogs and derivatives of a previously studied sigma (σ) receptor ligand, was synthesized and evaluated at σ and opioid receptors for receptor selectivity. The results of this study identified several compounds with nanomolar affinity at both σ receptor subtypes. Compounds 6 and 9 had the highest selectivity for both σ receptor subtypes, compared to μ opioid receptors. In addition, compounds 6 and 9 significantly reduced the convulsive effects of cocaine in mice, which would be consistent with antagonism of σ receptors.
Keywords: Antagonists; Opioid; Phenylpropyloxyethylaminesl; Sigma.
Copyright © 2013. Published by Elsevier Ltd.
Figures

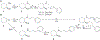
References
-
- Carrera MR; Meijler MM; Janda KD Bioorg. Med. Chem. 2004, 12, 5019. - PubMed
-
- Matsumoto RR; McCracken KA; Pouw B; Zhang Y; Bowen WD Neuropharmacology 2002, 42, 1043. - PubMed
-
- Maurice T; Martin-Fardon R; Romieu P; Matsumoto RR Neurosci. Biobehav. Rev. 2002, 26, 499. - PubMed
-
- Shibayama M; Ooi K; Johnson R; Scott B; Itoh Y. Curr. Genet. 2002, 42, 59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials

