HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation
- PMID: 23896681
- PMCID: PMC3828789
- DOI: 10.1161/JAHA.113.000264
HIV replication alters the composition of extrinsic pathway coagulation factors and increases thrombin generation
Abstract
Background: HIV infection leads to activation of coagulation, which may increase the risk for atherosclerosis and venous thromboembolic disease. We hypothesized that HIV replication increases coagulation potentially through alterations in extrinsic pathway factors.
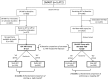
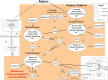
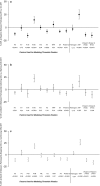
Methods and results: Extrinsic pathway factors were measured among a subset of HIV participants from the Strategies for Management of Anti-Retroviral Therapy (SMART) trial. Thrombin generation was estimated using validated computational modeling based on factor composition. We characterized the effect of antiretroviral therapy (ART) treatment versus the untreated state (HIV replication) via 3 separate analyses: (1) a cross-sectional comparison of those on and off ART (n=717); (2) a randomized comparison of deferring versus starting ART (n=217); and (3) a randomized comparison of stopping versus continuing ART (n=500). Compared with viral suppression, HIV replication consistently showed short-term increases in some procoagulants (eg, 15% to 23% higher FVIII; P<0.001) and decreases in key anticoagulants (eg, 5% to 9% lower antithrombin [AT] and 6% to 10% lower protein C; P<0.01). The net effect of HIV replication was to increase coagulation potential (eg, 24% to 48% greater thrombin generation from computational models; P<0.01 for all). The pattern of changes from HIV replication was reversed with ART treatment and consistent across all 3 independent comparisons.
Conclusions: HIV replication leads to complex changes in extrinsic pathway factors, with the net effect of increasing coagulation potential to a degree that may be clinically relevant. The key influence of changes in FVIII and AT suggests that HIV-related coagulation abnormalities may involve changes in hepatocyte function in the context of systemic inflammation.
Trial registration: ClinicalTrials.gov NCT00027352.
Keywords: HIV infection; HIV replication; antiretroviral therapy; coagulation; inflammation; thrombin generation.
Figures

References
-
- Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, Gatell J, Rakhmanova A, Johnson M, Kirk O, Lundgren J. Serious fatal and nonfatal non‐AIDS‐defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010; 55:262-270 - PubMed
-
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002; 8:1227-1234 - PubMed
-
- Taubes G. Does inflammation cut to the heart of the matter. Science. 2002; 296:242-245 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 TR000114/TR/NCATS NIH HHS/United States
- MC_U122886352/MRC_/Medical Research Council/United Kingdom
- U01-A1042170/PHS HHS/United States
- KL2 RR033182/RR/NCRR NIH HHS/United States
- U01-A1046362/PHS HHS/United States
- HL46703/HL/NHLBI NIH HHS/United States
- 5K12 RR023247/RR/NCRR NIH HHS/United States
- MC_UU_12023/16/MRC_/Medical Research Council/United Kingdom
- 5U01AI068641/AI/NIAID NIH HHS/United States
- P01 HL046703/HL/NHLBI NIH HHS/United States
- UM1 AI068641/AI/NIAID NIH HHS/United States
- K12 RR023247/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

