Identification of three novel natural product compounds that activate PXR and CAR and inhibit inflammation
- PMID: 23896737
- PMCID: PMC3771640
- DOI: 10.1007/s11095-013-1101-9
Identification of three novel natural product compounds that activate PXR and CAR and inhibit inflammation
Abstract
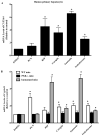
Purpose: To investigate the effects of three natural product compounds, carapin, santonin and isokobusone, on the activity of pregnane X receptor (PXR) and constitutive androstane receptor (CAR) in induction of drug-metabolizing enzymes and inhibition of inflammation.
Methods: The monkey kidney-derived fibroblast (CV-1) cells and human embryonic kidney HEK293 cells were used for transient transfection and luciferase reporter gene assays. Human primary hepatocytes and primary hepatocytes from wild type, PXR-/-, and hPXR transgenic mice were used to study the induction of drug-metabolizing enzymes and the implication of these compounds in inflammation.
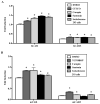
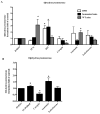
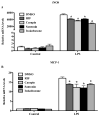
Results: Carapin, santonin and isokobusone activated both PXR and CAR in transient transfection and luciferase reporter gene assays. Mutagenesis studies showed that two amino acid residues, Phe305 of the rodent PXR and Leu308 of the human PXR, are critical for the recognition of these compounds by PXR. Importantly, the activation of PXR and CAR by these compounds induced the expression of drug-metabolizing enzymes in primary human and mouse hepatocytes. Furthermore, activation of PXR by these compounds inhibited the expression of inflammatory mediators in response to lipopolysaccharide (LPS). The effects of these natural compounds on drug metabolism and inflammation were abolished in PXR-/- hepatocytes.
Conclusions: Our results show that carapin, santonin and isokobusone activate PXR and CAR and induce drug-metabolizing enzymes. In addition, these compounds inhibited the expression of inflammatory mediators in response to LPS through the activation of PXR.
Figures
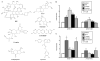


Similar articles
-
Differential activation of pregnane X receptor and constitutive androstane receptor by buprenorphine in primary human hepatocytes and HepG2 cells.J Pharmacol Exp Ther. 2010 Dec;335(3):562-71. doi: 10.1124/jpet.110.173187. Epub 2010 Sep 9. J Pharmacol Exp Ther. 2010. PMID: 20829393 Free PMC article.
-
Effects of atorvastatin metabolites on induction of drug-metabolizing enzymes and membrane transporters through human pregnane X receptor.Br J Pharmacol. 2012 Mar;165(5):1595-608. doi: 10.1111/j.1476-5381.2011.01665.x. Br J Pharmacol. 2012. PMID: 21913896 Free PMC article.
-
Modulation of constitutive androstane receptor (CAR) and pregnane X receptor (PXR) by 6-arylpyrrolo[2,1-d][1,5]benzothiazepine derivatives, ligands of peripheral benzodiazepine receptor (PBR).Toxicol Lett. 2011 Apr 25;202(2):148-54. doi: 10.1016/j.toxlet.2011.02.004. Epub 2011 Feb 18. Toxicol Lett. 2011. PMID: 21315811 Free PMC article.
-
Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator.Crit Rev Toxicol. 2014 Jan;44(1):64-82. doi: 10.3109/10408444.2013.835786. Epub 2013 Nov 4. Crit Rev Toxicol. 2014. PMID: 24180433 Free PMC article. Review.
-
CAR and PXR: the xenobiotic-sensing receptors.Steroids. 2007 Mar;72(3):231-46. doi: 10.1016/j.steroids.2006.12.006. Epub 2006 Dec 20. Steroids. 2007. PMID: 17284330 Free PMC article. Review.
Cited by
-
Salvia miltiorrhiza Bunge (Danshen) and Bioactive Compound Tanshinone IIA Alleviates Cisplatin-Induced Acute Kidney Injury Through Regulating PXR/NF-κB Signaling.Front Pharmacol. 2022 Mar 24;13:860383. doi: 10.3389/fphar.2022.860383. eCollection 2022. Front Pharmacol. 2022. PMID: 35401224 Free PMC article.
-
Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies.Nat Rev Drug Discov. 2016 Apr;15(4):249-74. doi: 10.1038/nrd.2015.3. Epub 2016 Jan 22. Nat Rev Drug Discov. 2016. PMID: 26794269 Review.
-
Dietary supplementation of gingerols- and shogaols-enriched ginger root extract attenuate pain-associated behaviors while modulating gut microbiota and metabolites in rats with spinal nerve ligation.J Nutr Biochem. 2022 Feb;100:108904. doi: 10.1016/j.jnutbio.2021.108904. Epub 2021 Nov 6. J Nutr Biochem. 2022. PMID: 34748918 Free PMC article.
-
Activation of pregnane X receptor sensitizes alcoholic steatohepatitis by transactivating fatty acid binding protein 4.Acta Pharm Sin B. 2024 Nov;14(11):4776-4788. doi: 10.1016/j.apsb.2024.08.029. Epub 2024 Sep 2. Acta Pharm Sin B. 2024. PMID: 39664417 Free PMC article.
-
Efficacy and compatibility mechanism of bear bile powder in Shexiang Tongxin dropping pills for acute myocardial infarction treatment.Chin Med. 2025 Jan 25;20(1):14. doi: 10.1186/s13020-025-01060-x. Chin Med. 2025. PMID: 39863867 Free PMC article.
References
-
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998 Jan 9;92(1):73–82. - PubMed
-
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000 Oct 19;407(6806):920–3. - PubMed
-
- Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999 Dec;56(6):1329–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN267200700004C/LM/NLM NIH HHS/United States
- DK083952/DK/NIDDK NIH HHS/United States
- CA014195/CA/NCI NIH HHS/United States
- N01-DK-7-0004/HHSN267200700004C/DK/NIDDK NIH HHS/United States
- R01 HD073070/HD/NICHD NIH HHS/United States
- P42 ES010337/ES/NIEHS NIH HHS/United States
- HHSN267200700004G/LM/NLM NIH HHS/United States
- ES019629/ES/NIEHS NIH HHS/United States
- R21 ES019629/ES/NIEHS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- ES010337/ES/NIEHS NIH HHS/United States
- HD073070/HD/NICHD NIH HHS/United States
- R01 DK083952/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

