Kaposi's sarcoma-associated herpesvirus ORF57 protein: exploiting all stages of viral mRNA processing
- PMID: 23896747
- PMCID: PMC3761232
- DOI: 10.3390/v5081901
Kaposi's sarcoma-associated herpesvirus ORF57 protein: exploiting all stages of viral mRNA processing
Abstract
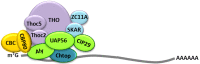
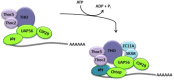
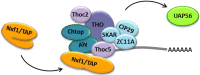
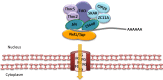
Nuclear mRNA export is a highly complex and regulated process in cells. Cellular transcripts must undergo successful maturation processes, including splicing, 5'-, and 3'-end processing, which are essential for assembly of an export competent ribonucleoprotein particle. Many viruses replicate in the nucleus of the host cell and require cellular mRNA export factors to efficiently export viral transcripts. However, some viral mRNAs undergo aberrant mRNA processing, thus prompting the viruses to express their own specific mRNA export proteins to facilitate efficient export of viral transcripts and allowing translation in the cytoplasm. This review will focus on the Kaposi's sarcoma-associated herpesvirus ORF57 protein, a multifunctional protein involved in all stages of viral mRNA processing and that is essential for virus replication. Using the example of ORF57, we will describe cellular bulk mRNA export pathways and highlight their distinct features, before exploring how the virus has evolved to exploit these mechanisms.
Figures
Similar articles
-
Recruitment of the complete hTREX complex is required for Kaposi's sarcoma-associated herpesvirus intronless mRNA nuclear export and virus replication.PLoS Pathog. 2008 Oct;4(10):e1000194. doi: 10.1371/journal.ppat.1000194. Epub 2008 Oct 31. PLoS Pathog. 2008. PMID: 18974867 Free PMC article.
-
Binding of cellular export factor REF/Aly by Kaposi's sarcoma-associated herpesvirus (KSHV) ORF57 protein is not required for efficient KSHV lytic replication.J Virol. 2012 Sep;86(18):9866-74. doi: 10.1128/JVI.01190-12. Epub 2012 Jul 3. J Virol. 2012. PMID: 22761374 Free PMC article.
-
The evolutionarily conserved Kaposi's sarcoma-associated herpesvirus ORF57 protein interacts with REF protein and acts as an RNA export factor.J Biol Chem. 2004 Jul 30;279(31):33001-11. doi: 10.1074/jbc.M313008200. Epub 2004 May 20. J Biol Chem. 2004. PMID: 15155762
-
Kaposi's sarcoma-associated herpesvirus ORF57 in viral RNA processing.Front Biosci (Landmark Ed). 2009 Jan 1;14(4):1516-28. doi: 10.2741/3322. Front Biosci (Landmark Ed). 2009. PMID: 19273144 Free PMC article. Review.
-
The KSHV RNA regulator ORF57: target specificity and its role in the viral life cycle.Wiley Interdiscip Rev RNA. 2016 Mar-Apr;7(2):173-85. doi: 10.1002/wrna.1323. Epub 2016 Jan 14. Wiley Interdiscip Rev RNA. 2016. PMID: 26769399 Review.
Cited by
-
A novel mechanism inducing genome instability in Kaposi's sarcoma-associated herpesvirus infected cells.PLoS Pathog. 2014 May 1;10(5):e1004098. doi: 10.1371/journal.ppat.1004098. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24788796 Free PMC article.
-
The Interplay between KSHV Infection and DNA-Sensing Pathways.Viruses. 2024 May 8;16(5):749. doi: 10.3390/v16050749. Viruses. 2024. PMID: 38793630 Free PMC article. Review.
-
Interactions between KSHV ORF57 and the novel human TREX proteins, CHTOP and CIP29.J Gen Virol. 2016 Aug;97(8):1904-1910. doi: 10.1099/jgv.0.000503. Epub 2016 May 16. J Gen Virol. 2016. PMID: 27189710 Free PMC article.
-
ORF57 overcomes the detrimental sequence bias of Kaposi's sarcoma-associated herpesvirus lytic genes.J Virol. 2015 May;89(9):5097-109. doi: 10.1128/JVI.03264-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694606 Free PMC article.
-
Hsp70 Isoforms Are Essential for the Formation of Kaposi's Sarcoma-Associated Herpesvirus Replication and Transcription Compartments.PLoS Pathog. 2015 Nov 20;11(11):e1005274. doi: 10.1371/journal.ppat.1005274. eCollection 2015 Nov. PLoS Pathog. 2015. PMID: 26587836 Free PMC article.
References
-
- Soulier J., Grollet L., Oksenhendler E., Cacoub P., Cazals-Hatem D., Babinet P., d’Agay M.F., Clauvel J.P., Raphael M., Degos L., et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. - PubMed
-
- Chang Y., Cesarman E., Pessin M.S., Lee F., Culpepper J., Knowles D.M., Moore P.S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

