Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes
- PMID: 23896970
- PMCID: PMC3788627
- DOI: 10.1369/0022155413501677
Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes
Abstract
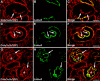
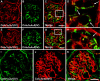
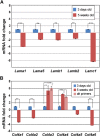
Kidney glomerular basement membranes (GBMs) undergo laminin and type IV collagen isoform substitutions during glomerular development, which are believed to be required for maturation of the filtration barrier. Specifically, GBMs of earliest glomeruli contain laminin α1β1γ1 and collagen α1α2α1(IV), whereas mature glomeruli contain laminin α5β2γ1 and collagen α3α4α5(IV). Here, we used confocal microscopy to simultaneously evaluate expression of different laminin and collagen IV isoforms in newborn mouse GBMs. Our results show loss of laminin α1 from GBMs in early capillary loop stages and continuous linear deposition of laminin bearing the α5 chain thereafter. In contrast, collagen α1α2α1(IV) persisted in linear patterns into late capillary loop stages, when collagen α3α4α5(IV) first appeared in discontinuous, non-linear patterns. This patchy pattern for collagen α3α4α5(IV) continued into maturing glomeruli where there were lengths of linear, laminin α5-positive GBM entirely lacking either isoform of collagen IV. Relative abundance of laminin and collagen IV mRNAs in newborn and 5-week-old mouse kidneys also differed, with those encoding laminin α1, α5, β1, β2, and γ1, and collagen α1(IV) and α2(IV) chains all significantly declining at 5 weeks, but α3(IV) and α4(IV) were significantly upregulated. We conclude that different biosynthetic mechanisms control laminin and type IV collagen expression in developing glomeruli.
Keywords: glomerular endothelial cells; glomerular filtration barrier; podocytes.
Conflict of interest statement
Figures





References
-
- Abrahamson DR, Irwin MH, St.John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR. 1989. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 109:3477–3491 - PMC - PubMed
-
- Abrahamson DR, St.John PL. 1992. Loss of laminin epitopes during glomerular basement membrane assembly in developing mouse kidneys. J Histochem Cytochem. 40:1943–1953 - PubMed
-
- Abrahamson DR, St.John PL, Isom K, Robert B, Miner JH. 2007. Partial rescue of glomerular laminin α5 mutations by wild-type endothelia produce hybrid glomeruli. J Am Soc Nephrol. 18:2285–2293 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

