Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions
- PMID: 23896987
- PMCID: PMC3773575
- DOI: 10.1038/cr.2013.102
Highly efficient induction and long-term maintenance of multipotent cardiovascular progenitors from human pluripotent stem cells under defined conditions
Abstract
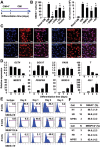
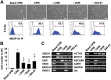
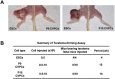
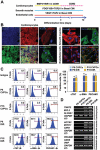
Cardiovascular progenitor cells (CVPCs) derived from human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), hold great promise for the study of cardiovascular development and cell-based therapy of heart diseases, but their applications are challenged by the difficulties in their efficient generation and stable maintenance. This study aims to develop chemically defined systems for robust generation and stable propagation of hPSC-derived CVPCs by modulating the key early developmental pathways involved in human cardiovascular specification and CVPC self-renewal. Herein we report that a combination of bone morphogenetic protein 4 (BMP4), glycogen synthase kinase 3 (GSK3) inhibitor CHIR99021 and ascorbic acid is sufficient to rapidly convert monolayer-cultured hPSCs, including hESCs and hiPSCs, into homogeneous CVPCs in a chemically defined medium under feeder- and serum-free culture conditions. These CVPCs stably self-renewed under feeder- and serum-free conditions and expanded over 10(7)-fold when the differentiation-inducing signals from BMP, GSK3 and Activin/Nodal pathways were simultaneously eliminated. Furthermore, these CVPCs exhibited expected genome-wide molecular features of CVPCs, retained potentials to generate major cardiovascular lineages including cardiomyocytes, smooth muscle cells and endothelial cells in vitro, and were non-tumorigenic in vivo. Altogether, the established systems reported here permit efficient generation and stable maintenance of hPSC-derived CVPCs, which represent a powerful tool to study early embryonic cardiovascular development and provide a potentially safe source of cells for myocardial regenerative medicine.
Figures


References
-
- Blin G, Neri T, Stefanovic S, Puceat M. Human embryonic and induced pluripotent stem cells in basic and clinical research in cardiology. Curr Stem Cell Res Ther. 2010;5:215–226. - PubMed
-
- Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. - PubMed
-
- Kattman SJ, Witty AD, Gagliardi M, et al. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

