Drosophila Myc integrates multiple signaling pathways to regulate intestinal stem cell proliferation during midgut regeneration
- PMID: 23896988
- PMCID: PMC3760623
- DOI: 10.1038/cr.2013.101
Drosophila Myc integrates multiple signaling pathways to regulate intestinal stem cell proliferation during midgut regeneration
Abstract
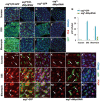
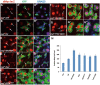
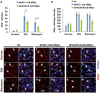
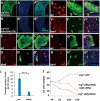
Intestinal stem cells (ISCs) in the Drosophila adult midgut are essential for maintaining tissue homeostasis, and their proliferation and differentiation speed up in order to meet the demand for replenishing the lost cells in response to injury. Several signaling pathways including JAK-STAT, EGFR and Hippo (Hpo) pathways have been implicated in damage-induced ISC proliferation, but the mechanisms that integrate these pathways have remained elusive. Here, we demonstrate that the Drosophila homolog of the oncoprotein Myc (dMyc) functions downstream of these signaling pathways to mediate their effects on ISC proliferation. dMyc expression in precursor cells is stimulated in response to tissue damage, and dMyc is essential for accelerated ISC proliferation and midgut regeneration. We show that tissue damage caused by dextran sulfate sodium feeding stimulates dMyc expression via the Hpo pathway, whereas bleomycin feeding activates dMyc through the JAK-STAT and EGFR pathways. We provide evidence that dMyc expression is transcriptionally upregulated by multiple signaling pathways, which is required for optimal ISC proliferation in response to tissue damage. We have also obtained evidence that tissue damage can upregulate dMyc expression post-transcriptionally. Finally, we show that a basal level of dMyc expression is required for ISC maintenance, proliferation and lineage differentiation during normal tissue homeostasis.
Figures



Similar articles
-
Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways.Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21064-9. doi: 10.1073/pnas.1012759107. Epub 2010 Nov 15. Proc Natl Acad Sci U S A. 2010. PMID: 21078993 Free PMC article.
-
Overexpression of dJmj differentially affects intestinal stem cells and differentiated enterocytes.Cell Signal. 2018 Jan;42:194-210. doi: 10.1016/j.cellsig.2017.10.017. Epub 2017 Nov 2. Cell Signal. 2018. PMID: 29102770
-
Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut.Development. 2012 Dec;139(24):4524-35. doi: 10.1242/dev.078261. Development. 2012. PMID: 23172913
-
Intestinal stem cell response to injury: lessons from Drosophila.Cell Mol Life Sci. 2016 Sep;73(17):3337-49. doi: 10.1007/s00018-016-2235-9. Epub 2016 May 2. Cell Mol Life Sci. 2016. PMID: 27137186 Free PMC article. Review.
-
Hippo pathway in intestinal homeostasis and tumorigenesis.Protein Cell. 2012 Apr;3(4):305-10. doi: 10.1007/s13238-012-2913-9. Epub 2012 Apr 10. Protein Cell. 2012. PMID: 22492181 Free PMC article. Review.
Cited by
-
Gilgamesh (Gish)/CK1γ regulates tissue homeostasis and aging in adult Drosophila midgut.J Cell Biol. 2020 Apr 6;219(4):e201909103. doi: 10.1083/jcb.201909103. J Cell Biol. 2020. PMID: 32328627 Free PMC article.
-
Controlling the Master: Chromatin Dynamics at the MYC Promoter Integrate Developmental Signaling.Genes (Basel). 2017 Apr 11;8(4):118. doi: 10.3390/genes8040118. Genes (Basel). 2017. PMID: 28398229 Free PMC article. Review.
-
Fear-of-intimacy-mediated zinc transport is required for Drosophila fat body endoreplication.BMC Biol. 2023 Apr 17;21(1):88. doi: 10.1186/s12915-023-01588-0. BMC Biol. 2023. PMID: 37069617 Free PMC article.
-
Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut.Elife. 2014 Mar 11;3:e01857. doi: 10.7554/eLife.01857. Elife. 2014. PMID: 24618900 Free PMC article.
-
Gene expression profiling identifies the zinc-finger protein Charlatan as a regulator of intestinal stem cells in Drosophila.Development. 2014 Jul;141(13):2621-32. doi: 10.1242/dev.106237. Development. 2014. PMID: 24961799 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

