Spike timing-dependent plasticity at GABAergic synapses in the ventral tegmental area
- PMID: 23897235
- PMCID: PMC3800449
- DOI: 10.1113/jphysiol.2013.257873
Spike timing-dependent plasticity at GABAergic synapses in the ventral tegmental area
Abstract
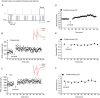
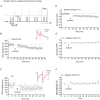
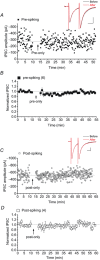
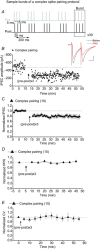
Persistent changes in excitatory and inhibitory synaptic strengths to the ventral tegmental area (VTA) dopamine (DA) neurons in response to addictive drugs may underlie the transition from casual to compulsive drug use. While an enormous amount of work has been done in the area of glutamatergic plasticity of the VTA, little is known regarding the learning rules governing GABAergic plasticity in the VTA. Spike timing-dependent plasticity, STDP, has attracted considerable attention primarily due to its potential roles in processing and storage of information in the brain and there is emerging evidence for the existence of STDP at inhibitory synapses. We therefore used whole-cell recordings in rat midbrain slices to investigate whether near-coincident pre- and postsynaptic firing induces a lasting change in synaptic efficacy of VTA GABAergic synapses. We found that a Hebbian form of STDP including long-term potentiation (LTP) and long-term depression (LTD) can be induced at GABAergic synapses onto VTA DA neurons and relies on the precise temporal order of pre- and postsynaptic spiking. Importantly, GABAergic STDP is heterosynaptic (NMDA receptor dependent): triggered by correlated activities of the presynaptic glutamatergic input and postsynaptic DA cells. GABAergic STDP is postsynaptic and has an associative component since pre- or postsynaptic spiking per se did not induce STDP. STDP of GABAergic synapses in the VTA provides physiologically relevant forms of inhibitory plasticity that may underlie natural reinforcement of reward-related behaviours. Moreover, this form of inhibitory plasticity may mediate some of the reinforcing, aversive and addictive properties of drugs of abuse.
Figures

Similar articles
-
Dopamine Receptor Activation Is Required for GABAergic Spike Timing-Dependent Plasticity in Response to Complex Spike Pairing in the Ventral Tegmental Area.Front Synaptic Neurosci. 2018 Sep 21;10:32. doi: 10.3389/fnsyn.2018.00032. eCollection 2018. Front Synaptic Neurosci. 2018. PMID: 30297996 Free PMC article.
-
Morphine-induced modulation of LTD at GABAergic synapses in the ventral tegmental area.Neuropharmacology. 2011 Dec;61(7):1166-71. doi: 10.1016/j.neuropharm.2010.11.012. Epub 2010 Dec 1. Neuropharmacology. 2011. PMID: 21129388
-
Dopamine-enabled anti-Hebbian timing-dependent plasticity in prefrontal circuitry.Front Neural Circuits. 2014 Apr 23;8:38. doi: 10.3389/fncir.2014.00038. eCollection 2014. Front Neural Circuits. 2014. PMID: 24795571 Free PMC article.
-
LTP of GABAergic synapses in the ventral tegmental area and beyond.J Physiol. 2008 Mar 15;586(6):1487-93. doi: 10.1113/jphysiol.2007.148098. Epub 2007 Dec 13. J Physiol. 2008. PMID: 18079157 Free PMC article. Review.
-
Dopamine and reward seeking: the role of ventral tegmental area.Rev Neurosci. 2014;25(5):621-30. doi: 10.1515/revneuro-2014-0019. Rev Neurosci. 2014. PMID: 24887956 Review.
Cited by
-
Excitatory and inhibitory STDP jointly tune feedforward neural circuits to selectively propagate correlated spiking activity.Front Comput Neurosci. 2014 May 7;8:53. doi: 10.3389/fncom.2014.00053. eCollection 2014. Front Comput Neurosci. 2014. PMID: 24847242 Free PMC article.
-
Coexistence of Multiple Types of Synaptic Plasticity in Individual Hippocampal CA1 Pyramidal Neurons.Front Synaptic Neurosci. 2017 Mar 14;9:7. doi: 10.3389/fnsyn.2017.00007. eCollection 2017. Front Synaptic Neurosci. 2017. PMID: 28352224 Free PMC article.
-
Convergent Neuronal Plasticity and Metaplasticity Mechanisms of Stress, Nicotine, and Alcohol.Annu Rev Pharmacol Toxicol. 2018 Jan 6;58:547-566. doi: 10.1146/annurev-pharmtox-010617-052735. Epub 2017 Oct 4. Annu Rev Pharmacol Toxicol. 2018. PMID: 28977763 Free PMC article. Review.
-
VTA dopamine neuron plasticity - the unusual suspects.Eur J Neurosci. 2016 Dec;44(12):2975-2983. doi: 10.1111/ejn.13425. Epub 2016 Nov 1. Eur J Neurosci. 2016. PMID: 27711998 Free PMC article. Review.
-
Ethanol blocks a novel form of iLTD, but not iLTP of inhibitory inputs to VTA GABA neurons.Neuropsychopharmacology. 2023 Aug;48(9):1396-1408. doi: 10.1038/s41386-023-01554-y. Epub 2023 Mar 10. Neuropsychopharmacology. 2023. PMID: 36899030 Free PMC article.
References
-
- Caporale N, Dan Y. Spike timing-dependent plasticity: a Hebbian learning rule. Annu Rev Neurosci. 2008;31:25–46. - PubMed
-
- Chergui K, Charlety PJ, Akaoka H, Saunier CF, Brunet JL, Buda M, Svensson TH, Chouvet G. Tonic activation of NMDA receptors causes spontaneous burst discharge of rat midbrain dopamine neurons in vivo. Eur J Neurosci. 1993;5:137–144. - PubMed
-
- Creed MC, Luscher C. Drug-evoked synaptic plasticity: beyond metaplasticity. Curr Opin Neurobiol. 2013;23:553–558. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

