Dissection of the role of pili and type 2 and 3 secretion systems in adherence and biofilm formation of an atypical enteropathogenic Escherichia coli strain
- PMID: 23897608
- PMCID: PMC3811761
- DOI: 10.1128/IAI.00620-13
Dissection of the role of pili and type 2 and 3 secretion systems in adherence and biofilm formation of an atypical enteropathogenic Escherichia coli strain
Abstract
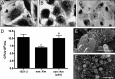
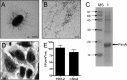
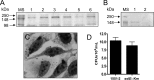
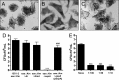
Atypical enteropathogenic Escherichia coli (aEPEC) strains are diarrheal pathogens that lack bundle-forming pilus production but possess the virulence-associated locus of enterocyte effacement. aEPEC strain 1551-2 produces localized adherence (LA) on HeLa cells; however, its isogenic intimin (eae) mutant produces a diffuse-adherence (DA) pattern. In this study, we aimed to identify the DA-associated adhesin of the 1551-2 eae mutant. Electron microscopy of 1551-2 identified rigid rod-like pili composed of an 18-kDa protein, which was identified as the major pilin subunit of type 1 pilus (T1P) by mass spectrometry analysis. Deletion of fimA in 1551-2 affected biofilm formation but had no effect on adherence properties. Analysis of secreted proteins in supernatants of this strain identified a 150-kDa protein corresponding to SslE, a type 2 secreted protein that was recently reported to be involved in biofilm formation of rabbit and human EPEC strains. However, neither adherence nor biofilm formation was affected in a 1551-2 sslE mutant. We then investigated the role of the EspA filament associated with the type 3 secretion system (T3SS) in DA by generating a double eae espA mutant. This strain was no longer adherent, strongly suggesting that the T3SS translocon is the DA adhesin. In agreement with these results, specific anti-EspA antibodies blocked adherence of the 1551-2 eae mutant. Our data support a role for intimin in LA, for the T3SS translocon in DA, and for T1P in biofilm formation, all of which may act in concert to facilitate host intestinal colonization by aEPEC strains.
Figures

Similar articles
-
Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin.Microbiology (Reading). 2004 Mar;150(Pt 3):527-538. doi: 10.1099/mic.0.26740-0. Microbiology (Reading). 2004. PMID: 14993302
-
The Escherichia coli common pilus and the bundle-forming pilus act in concert during the formation of localized adherence by enteropathogenic E. coli.J Bacteriol. 2009 Jun;191(11):3451-61. doi: 10.1128/JB.01539-08. Epub 2009 Feb 13. J Bacteriol. 2009. PMID: 19218393 Free PMC article.
-
The localized adherence pattern of an atypical enteropathogenic Escherichia coli is mediated by intimin omicron and unexpectedly promotes HeLa cell invasion.Cell Microbiol. 2008 Feb;10(2):415-25. doi: 10.1111/j.1462-5822.2007.01054.x. Epub 2007 Oct 2. Cell Microbiol. 2008. PMID: 17910741
-
Enteropathogenic Escherichia coli: foe or innocent bystander?Clin Microbiol Infect. 2015 Aug;21(8):729-34. doi: 10.1016/j.cmi.2015.01.015. Epub 2015 Jan 28. Clin Microbiol Infect. 2015. PMID: 25726041 Free PMC article. Review.
-
Proteinaceous determinants of surface colonization in bacteria: bacterial adhesion and biofilm formation from a protein secretion perspective.Front Microbiol. 2013 Oct 14;4:303. doi: 10.3389/fmicb.2013.00303. Front Microbiol. 2013. PMID: 24133488 Free PMC article. Review.
Cited by
-
Escherichia albertii Pathogenesis.EcoSal Plus. 2020 Jun;9(1):10.1128/ecosalplus.ESP-0015-2019. doi: 10.1128/ecosalplus.ESP-0015-2019. EcoSal Plus. 2020. PMID: 32588811 Free PMC article. Review.
-
Differential Regulation of the Surface-Exposed and Secreted SslE Lipoprotein in Extraintestinal Pathogenic Escherichia coli.PLoS One. 2016 Sep 6;11(9):e0162391. doi: 10.1371/journal.pone.0162391. eCollection 2016. PLoS One. 2016. PMID: 27598999 Free PMC article.
-
Genetic and Antimicrobial Resistance Profiles of Mammary Pathogenic E. coli (MPEC) Isolates from Bovine Clinical Mastitis.Pathogens. 2022 Nov 28;11(12):1435. doi: 10.3390/pathogens11121435. Pathogens. 2022. PMID: 36558768 Free PMC article.
-
The Fis Nucleoid Protein Negatively Regulates the Phase Variation fimS Switch of the Type 1 Pilus Operon in Enteropathogenic Escherichia coli.Front Microbiol. 2022 Apr 28;13:882563. doi: 10.3389/fmicb.2022.882563. eCollection 2022. Front Microbiol. 2022. PMID: 35572706 Free PMC article.
-
Diarrheagenic Escherichia coli.Braz J Microbiol. 2016 Dec;47 Suppl 1(Suppl 1):3-30. doi: 10.1016/j.bjm.2016.10.015. Epub 2016 Nov 5. Braz J Microbiol. 2016. PMID: 27866935 Free PMC article. Review.
References
-
- Kaper JB. 1996. Defining EPEC. Rev. Microbiol. 27:130–133
-
- Gomes TAT, Rassi V, MacDonald KL, Ramos SR, Trabulsi LR, Vieira MAM, Guth BE, Candeias JA, Ivey C, Toledo MR, Blake PA. 1991. Enteropathogens associated with acute diarrheal disease in urban infants in São Paulo, Brazil. J. Infect. Dis. 164:331–337 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

