A chimeric Plasmodium falciparum merozoite surface protein vaccine induces high titers of parasite growth inhibitory antibodies
- PMID: 23897613
- PMCID: PMC3811772
- DOI: 10.1128/IAI.00522-13
A chimeric Plasmodium falciparum merozoite surface protein vaccine induces high titers of parasite growth inhibitory antibodies
Abstract
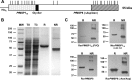
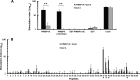
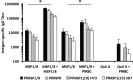
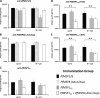
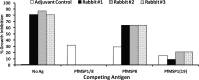
The C-terminal 19-kDa domain of Plasmodium falciparum merozoite surface protein 1 (PfMSP119) is an established target of protective antibodies. However, clinical trials of PfMSP142, a leading blood-stage vaccine candidate which contains the protective epitopes of PfMSP119, revealed suboptimal immunogenicity and efficacy. Based on proof-of-concept studies in the Plasmodium yoelii murine model, we produced a chimeric vaccine antigen containing recombinant PfMSP119 (rPfMSP119) fused to the N terminus of P. falciparum merozoite surface protein 8 that lacked its low-complexity Asn/Asp-rich domain, rPfMSP8 (ΔAsn/Asp). Immunization of mice with the chimeric rPfMSP1/8 vaccine elicited strong T cell responses to conserved epitopes associated with the rPfMSP8 (ΔAsn/Asp) fusion partner. While specific for PfMSP8, this T cell response was adequate to provide help for the production of high titers of antibodies to both PfMSP119 and rPfMSP8 (ΔAsn/Asp) components. This occurred with formulations adjuvanted with either Quil A or with Montanide ISA 720 plus CpG oligodeoxynucleotide (ODN) and was observed in both inbred and outbred strains of mice. PfMSP1/8-induced antibodies were highly reactive with two major alleles of PfMSP119 (FVO and 3D7). Of particular interest, immunization with PfMSP1/8 elicited higher titers of PfMSP119-specific antibodies than a combined formulation of rPfMSP142 and rPfMSP8 (ΔAsn/Asp). As a measure of functionality, PfMSP1/8-specific rabbit IgG was shown to potently inhibit the in vitro growth of blood-stage parasites of the FVO and 3D7 strains of P. falciparum. These data support the further testing and evaluation of this chimeric PfMSP1/8 antigen as a component of a multivalent vaccine for P. falciparum malaria.
Figures
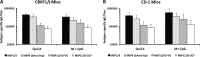
Similar articles
-
Immunogenicity of a chimeric Plasmodium falciparum merozoite surface protein vaccine in Aotus monkeys.Malar J. 2016 Mar 15;15:159. doi: 10.1186/s12936-016-1226-5. Malar J. 2016. PMID: 26975721 Free PMC article.
-
Evaluation of the immunogenicity and vaccine potential of recombinant Plasmodium falciparum merozoite surface protein 8.Infect Immun. 2012 Jul;80(7):2473-84. doi: 10.1128/IAI.00211-12. Epub 2012 May 14. Infect Immun. 2012. PMID: 22585960 Free PMC article.
-
Inclusion of an Optimized Plasmodium falciparum Merozoite Surface Protein 2-Based Antigen in a Trivalent, Multistage Malaria Vaccine.J Immunol. 2021 Apr 15;206(8):1817-1831. doi: 10.4049/jimmunol.2000927. Epub 2021 Mar 31. J Immunol. 2021. PMID: 33789984 Free PMC article.
-
Evaluation of a Plasmodium-Specific Carrier Protein To Enhance Production of Recombinant Pfs25, a Leading Transmission-Blocking Vaccine Candidate.Infect Immun. 2017 Dec 19;86(1):e00486-17. doi: 10.1128/IAI.00486-17. Print 2018 Jan. Infect Immun. 2017. PMID: 28993460 Free PMC article.
-
Merozoite surface protein 1, immune evasion, and vaccines against asexual blood stage malaria.Parassitologia. 1999 Sep;41(1-3):409-14. Parassitologia. 1999. PMID: 10697894 Review.
Cited by
-
Neutralizing and interfering human antibodies define the structural and mechanistic basis for antigenic diversion.Nat Commun. 2022 Oct 6;13(1):5888. doi: 10.1038/s41467-022-33336-3. Nat Commun. 2022. PMID: 36202833 Free PMC article.
-
Na+ Influx Induced by New Antimalarials Causes Rapid Alterations in the Cholesterol Content and Morphology of Plasmodium falciparum.PLoS Pathog. 2016 May 26;12(5):e1005647. doi: 10.1371/journal.ppat.1005647. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27227970 Free PMC article.
-
Vaccines to combat river blindness: expression, selection and formulation of vaccines against infection with Onchocerca volvulus in a mouse model.Int J Parasitol. 2014 Aug;44(9):637-46. doi: 10.1016/j.ijpara.2014.04.006. Epub 2014 Jun 5. Int J Parasitol. 2014. PMID: 24907553 Free PMC article.
-
Recent advances in recombinant protein-based malaria vaccines.Vaccine. 2015 Dec 22;33(52):7433-43. doi: 10.1016/j.vaccine.2015.09.093. Epub 2015 Oct 11. Vaccine. 2015. PMID: 26458807 Free PMC article. Review.
-
The Cellular and Molecular Interaction Between Erythrocytes and Plasmodium falciparum Merozoites.Front Cell Infect Microbiol. 2022 Mar 31;12:816574. doi: 10.3389/fcimb.2022.816574. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35433504 Free PMC article. Review.
References
-
- Chan M. 2012. World malaria report 2012. World Health Organization, Geneva, Switzerland
-
- Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Machevo S, Acacio S, Bulo H, Sigauque B, Macete E, Alonso P, Abdulla S, Salim N, Minja R, Mpina M, Ahmed S, Ali AM, Mtoro AT, Hamad AS, Mutani P, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Bihoun B, Guiraud I, Kabore B, Sombie O, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Oneko M, Odero C, Otieno K, Awino N, McMorrow M, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Otsyula N, Gondi S, Otieno A, Owira V, Oguk E, Odongo G, Woods JB, Ogutu B, Njuguna P, Chilengi R, Akoo P, Kerubo C, Maingi C, Lang T, Olotu A, Bejon P, Marsh K, Mwambingu G, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Dosoo D, Asante I, Adjei G, Kwara E, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Mahende C, Liheluka E, Malle L, Lemnge M, Theander TG, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Sarfo A, Agyekum A, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Tembo T, Tegha G, Tsidya M, Kilembe J, Chawinga C, Ballou WR, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Olivier A, Vekemans J, Carter T, Kaslow D, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P. 2012. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367:2284–2295 - PMC - PubMed
-
- Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmuller B, Issifou S, Kremsner PG, Sacarlal J, Aide P, Lanaspa M, Aponte JJ, Nhamuave A, Quelhas D, Bassat Q, Mandjate S, Macete E, Alonso P, Abdulla S, Salim N, Juma O, Shomari M, Shubis K, Machera F, Hamad AS, Minja R, Mtoro A, Sykes A, Ahmed S, Urassa AM, Ali AM, Mwangoka G, Tanner M, Tinto H, D'Alessandro U, Sorgho H, Valea I, Tahita MC, Kabore W, Ouedraogo S, Sandrine Y, Guiguemde RT, Ouedraogo JB, Hamel MJ, Kariuki S, Odero C, Oneko M, Otieno K, Awino N, Omoto J, Williamson J, Muturi-Kioi V, Laserson KF, Slutsker L, Otieno W, Otieno L, Nekoye O, Gondi S, Otieno A, Ogutu B, Wasuna R, Owira V, Jones D, Onyango AA, Njuguna P, Chilengi R, Akoo P, Kerubo C, Gitaka J, Maingi C, Lang T, Olotu A, Tsofa B, Bejon P, Peshu N, Marsh K, Owusu-Agyei S, Asante KP, Osei-Kwakye K, Boahen O, Ayamba S, Kayan K, Owusu-Ofori R, Dosoo D, Asante I, Adjei G, Chandramohan D, Greenwood B, Lusingu J, Gesase S, Malabeja A, Abdul O, Kilavo H, Mahende C, Liheluka E, Lemnge M, Theander T, Drakeley C, Ansong D, Agbenyega T, Adjei S, Boateng HO, Rettig T, Bawa J, Sylverken J, Sambian D, Agyekum A, Owusu L, Martinson F, Hoffman I, Mvalo T, Kamthunzi P, Nkomo R, Msika A, Jumbe A, Chome N, Nyakuipa D, Chintedza J, Ballou WR, Bruls M, Cohen J, Guerra Y, Jongert E, Lapierre D, Leach A, Lievens M, Ofori-Anyinam O, Vekemans J, Carter T, Leboulleux D, Loucq C, Radford A, Savarese B, Schellenberg D, Sillman M, Vansadia P. 2011. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 365:1863–1875 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

