Combined stimulation of Toll-like receptor 5 and NOD1 strongly potentiates activity of NF-κB, resulting in enhanced innate immune reactions and resistance to Salmonella enterica serovar Typhimurium infection
- PMID: 23897616
- PMCID: PMC3811746
- DOI: 10.1128/IAI.00525-13
Combined stimulation of Toll-like receptor 5 and NOD1 strongly potentiates activity of NF-κB, resulting in enhanced innate immune reactions and resistance to Salmonella enterica serovar Typhimurium infection
Abstract
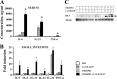
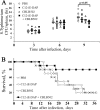
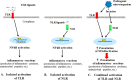
Pathogen recognition receptors (PRRs) are essential components of host innate immune systems that detect specific conserved pathogen-associated molecular patterns (PAMPs) presented by microorganisms. Members of two families of PRRs, transmembrane Toll-like receptors (TLRs 1, 2, 4, 5, and 6) and cytosolic NOD receptors (NOD1 and NOD2), are stimulated upon recognition of various bacterial PAMPs. Such stimulation leads to induction of a number of immune defense reactions, mainly triggered via activation of the transcription factor NF-κB. While coordination of responses initiated via different PRRs sensing multiple PAMPS present during an infection makes clear biological sense for the host, such interactions have not been fully characterized. Here, we demonstrate that combined stimulation of NOD1 and TLR5 (as well as other NOD and TLR family members) strongly potentiates activity of NF-κB and induces enhanced levels of innate immune reactions (e.g., cytokine production) both in vitro and in vivo. Moreover, we show that an increased level of NF-κB activity plays a critical role in formation of downstream responses. In live mice, synergy between these receptors resulting in potentiation of NF-κB activity was organ specific, being most prominent in the gastrointestinal tract. Coordinated activity of NOD1 and TLR5 significantly increased protection of mice against enteroinvasive Salmonella infection. Obtained results suggest that cooperation of NOD and TLR receptors is important for effective responses to microbial infection in vivo.
Figures

Comment in
-
Arginine cools the inflamed gut.Infect Immun. 2013 Oct;81(10):3500-2. doi: 10.1128/IAI.00789-13. Epub 2013 Jul 29. Infect Immun. 2013. PMID: 23897606 Free PMC article. No abstract available.
References
-
- Reiling N, Hölscher C, Fehrenbach A, Kröger S, Kirschning CJ, Goyert S, Ehlers S. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480–3484 - PubMed
-
- Takeuchi O, Hoshino K, Akira S. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392–5396 - PubMed
-
- Silva GK, Gutierrez FR, Guedes PM, Horta CV, Cunha LD, Mineo TW, Santiago-Silva J, Kobayashi KS, Flavell RA, Silva JS, Zamboni DS. 2010. Cutting edge: nucleotide-binding oligomerization domain 1-dependent responses account for murine resistance against Trypanosoma cruzi infection. J. Immunol. 184:1148–1152 - PubMed
-
- Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nuñez G, Flavell RA. 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307:731–734 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

