Temozolomide downregulates P-glycoprotein expression in glioblastoma stem cells by interfering with the Wnt3a/glycogen synthase-3 kinase/β-catenin pathway
- PMID: 23897632
- PMCID: PMC3813413
- DOI: 10.1093/neuonc/not104
Temozolomide downregulates P-glycoprotein expression in glioblastoma stem cells by interfering with the Wnt3a/glycogen synthase-3 kinase/β-catenin pathway
Abstract
Background: Glioblastoma multiforme stem cells display a highly chemoresistant phenotype, whose molecular basis is poorly known. We aim to clarify this issue and to investigate the effects of temozolomide on chemoresistant stem cells.
Methods: A panel of human glioblastoma cultures, grown as stem cells (neurospheres) and adherent cells, was used.
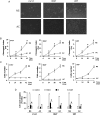
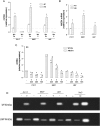
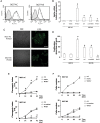
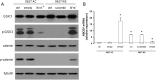
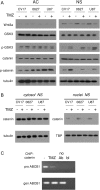
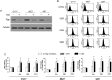
Results: Neurospheres had a multidrug resistant phenotype compared with adherent cells. Such chemoresistance was overcome by apparently noncytotoxic doses of temozolomide, which chemosensitized glioblastoma cells to doxorubicin, vinblastine, and etoposide. This effect was selective for P-glycoprotein (Pgp) substrates and for stem cells, leading to an investigation of whether there was a correlation between the expression of Pgp and the activity of typical stemness pathways. We found that Wnt3a and ABCB1, which encodes for Pgp, were both highly expressed in glioblastoma stem cells and reduced by temozolomide. Temozolomide-treated cells had increased methylation of the cytosine-phosphate-guanine islands in the Wnt3a gene promoter, decreased expression of Wnt3a, disrupted glycogen synthase-3 kinase/β-catenin axis, reduced transcriptional activation of ABCB1, and a lower amount and activity of Pgp. Wnt3a overexpression was sufficient to transform adherent cells into neurospheres and to simultaneously increase proliferation and ABCB1 expression. On the contrary, glioblastoma stem cells silenced for Wnt3a lost the ability to form neurospheres and reduced at the same time the proliferation rate and ABCB1 levels.
Conclusions: Our work suggests that Wnt3a is an autocrine mediator of stemness, proliferation, and chemoresistance in human glioblastoma and that temozolomide may chemosensitize the stem cell population by downregulating Wnt3a signaling.
Keywords: ABCB1/P-glycoprotein; Wnt3a; glioblastoma stem cells; temozolomide.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

