Bile formation and secretion
- PMID: 23897680
- PMCID: PMC4091928
- DOI: 10.1002/cphy.c120027
Bile formation and secretion
Abstract
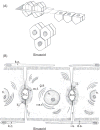
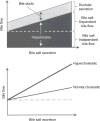
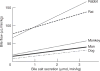
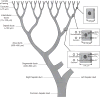
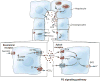
Bile is a unique and vital aqueous secretion of the liver that is formed by the hepatocyte and modified down stream by absorptive and secretory properties of the bile duct epithelium. Approximately 5% of bile consists of organic and inorganic solutes of considerable complexity. The bile-secretory unit consists of a canalicular network which is formed by the apical membrane of adjacent hepatocytes and sealed by tight junctions. The bile canaliculi (∼1 μm in diameter) conduct the flow of bile countercurrent to the direction of portal blood flow and connect with the canal of Hering and bile ducts which progressively increase in diameter and complexity prior to the entry of bile into the gallbladder, common bile duct, and intestine. Canalicular bile secretion is determined by both bile salt-dependent and independent transport systems which are localized at the apical membrane of the hepatocyte and largely consist of a series of adenosine triphosphate-binding cassette transport proteins that function as export pumps for bile salts and other organic solutes. These transporters create osmotic gradients within the bile canalicular lumen that provide the driving force for movement of fluid into the lumen via aquaporins. Species vary with respect to the relative amounts of bile salt-dependent and independent canalicular flow and cholangiocyte secretion which is highly regulated by hormones, second messengers, and signal transduction pathways. Most determinants of bile secretion are now characterized at the molecular level in animal models and in man. Genetic mutations serve to illuminate many of their functions.
© 2013 American Physiological Society.
Figures





References
-
- Abu-Hayyeh S, Martinez-Becerra P, Sheikh Abdul Kadir SH, Selden C, Romero MR, Rees M, Marschall HU, Marin JJ, Williamson C. Inhibition of Na+-taurocholate Co-transporting polypeptide-mediated bile acid transport by cholestatic sulfated progesterone metabolites. J Biol Chem. 2010;285:16504–16512. - PMC - PubMed
-
- Accatino L, Pizarro M, Solís N, Koenig CS, Vollrath V, Chianale J. Modulation of hepatic content and biliary excretion of P-glycoproteins in hepatocellular and obstructive cholestasis in the rat. J Hepatol. 1996;25:349–361. - PubMed
-
- Adachi Y, Kobuyashi H, Kurumi Y. ATP-dependent taurocholate transport by rat liver canalicular membrane vesicles. Hepatology. 1991;14:655–659. - PubMed
-
- Agre P, Bonhivers M, Borgnia MJ. The aquaporins, blueprints for cellular plumbing systems. J Biol Chem. 1998;273:14659–14662. - PubMed
-
- Akita H, Suzuki H, Hirohashi T, Takikawa H, Sugiyama Y. Transport activity of human MRP3 expressed in Sf9 cells: Comparative studies with rat MRP3. Pharm Res. 2002;19:34–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

