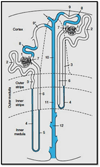
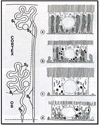
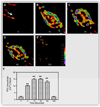
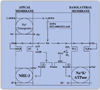
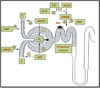
Proximal nephron
- PMID: 23897681
- PMCID: PMC3760239
- DOI: 10.1002/cphy.c110061
Proximal nephron
Abstract
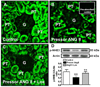
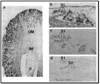
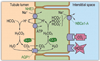
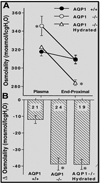
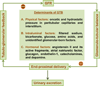
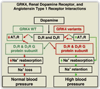
The kidney plays a fundamental role in maintaining body salt and fluid balance and blood pressure homeostasis through the actions of its proximal and distal tubular segments of nephrons. However, proximal tubules are well recognized to exert a more prominent role than distal counterparts. Proximal tubules are responsible for reabsorbing approximately 65% of filtered load and most, if not all, of filtered amino acids, glucose, solutes, and low molecular weight proteins. Proximal tubules also play a key role in regulating acid-base balance by reabsorbing approximately 80% of filtered bicarbonate. The purpose of this review article is to provide a comprehensive overview of new insights and perspectives into current understanding of proximal tubules of nephrons, with an emphasis on the ultrastructure, molecular biology, cellular and integrative physiology, and the underlying signaling transduction mechanisms. The review is divided into three closely related sections. The first section focuses on the classification of nephrons and recent perspectives on the potential role of nephron numbers in human health and diseases. The second section reviews recent research on the structural and biochemical basis of proximal tubular function. The final section provides a comprehensive overview of new insights and perspectives in the physiological regulation of proximal tubular transport by vasoactive hormones. In the latter section, attention is particularly paid to new insights and perspectives learnt from recent cloning of transporters, development of transgenic animals with knockout or knockin of a particular gene of interest, and mapping of signaling pathways using microarrays and/or physiological proteomic approaches.
© 2013 American Physiological Society.
Figures













References
-
- Abuladze N, Lee I, Newman D, Hwang J, Pushkin A, Kurtz I. Axial heterogeneity of sodium-bicarbonate cotransporter expression in the rabbit proximal tubule. Am J Physiol. 1998;274:F628–F633. - PubMed
-
- Adachi M, Yang YY, Furuichi Y, Miyamoto C. Cloning and characterization of cDNA encoding human A-type endothelin receptor. Biochem Biophys Res Commun. 1991;180:1265–1272. - PubMed
-
- Aizman O, Aperia A. Na,K-ATPase as a signal transducer. Ann N Y Acad Sci. 2003;986:489–496. 489–496. - PubMed
-
- Alpern RJ. Cell mechanisms of proximal tubule acidification. Physiol Rev. 1990;70:79–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

