Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence
- PMID: 23897750
- PMCID: PMC4377082
- DOI: 10.1002/stem.1488
Dysfunction of endothelial progenitor cells from smokers and chronic obstructive pulmonary disease patients due to increased DNA damage and senescence
Abstract
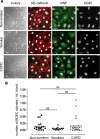
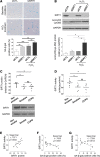
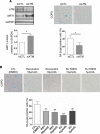
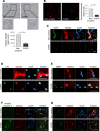
Cardiovascular disease (CVD) is a major cause of death in smokers, particularly in those with chronic obstructive pulmonary disease (COPD). Circulating endothelial progenitor cells (EPC) are required for endothelial homeostasis, and their dysfunction contributes to CVD. To investigate EPC dysfunction in smokers, we isolated and expanded blood outgrowth endothelial cells (BOEC) from peripheral blood samples from healthy nonsmokers, healthy smokers, and COPD patients. BOEC from smokers and COPD patients showed increased DNA double-strand breaks and senescence compared to nonsmokers. Senescence negatively correlated with the expression and activity of sirtuin-1 (SIRT1), a protein deacetylase that protects against DNA damage and cellular senescence. Inhibition of DNA damage response by silencing of ataxia telangiectasia mutated (ATM) kinase resulted in upregulation of SIRT1 expression and decreased senescence. Treatment of BOEC from COPD patients with the SIRT1 activator resveratrol or an ATM inhibitor (KU-55933) also rescued the senescent phenotype. Using an in vivo mouse model of angiogenesis, we demonstrated that senescent BOEC from COPD patients are dysfunctional, displaying impaired angiogenic ability and increased apoptosis compared to cells from healthy nonsmokers. Therefore, this study identifies epigenetic regulation of DNA damage and senescence as pathogenetic mechanisms linked to endothelial progenitors' dysfunction in smokers and COPD patients. These defects may contribute to vascular disease and cardiovascular events in smokers and could therefore constitute therapeutic targets for intervention.
Keywords: Ataxia telangiectasia-mutated kinase; Cellular senescence; DNA damage response; Endothelial progenitor cells; Sirtuin; Smoking.
© AlphaMed Press.
Figures


References
-
- Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Fuchs S, Ghanaati S, Orth C, et al. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30:526–534. - PubMed
-
- Hendrickx B, Verdonck K, Van den Berge S, et al. Integration of blood outgrowth endothelial cells in dermal fibroblast sheets promotes full thickness wound healing. Stem Cells. 2010;28:1165–1177. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

