Value of biomarkers in osteoarthritis: current status and perspectives
- PMID: 23897772
- PMCID: PMC3812859
- DOI: 10.1136/annrheumdis-2013-203726
Value of biomarkers in osteoarthritis: current status and perspectives
Erratum in
-
Correction.Ann Rheum Dis. 2017 Sep;76(9):e35. doi: 10.1136/annrheumdis-2013-203726corr1. Epub 2017 May 25. Ann Rheum Dis. 2017. PMID: 28546259 Free PMC article. No abstract available.
Abstract
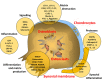
Osteoarthritis affects the whole joint structure with progressive changes in cartilage, menisci, ligaments and subchondral bone, and synovial inflammation. Biomarkers are being developed to quantify joint remodelling and disease progression. This article was prepared following a working meeting of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis convened to discuss the value of biochemical markers of matrix metabolism in drug development in osteoarthritis. The best candidates are generally molecules or molecular fragments present in cartilage, bone or synovium and may be specific to one type of joint tissue or common to them all. Many currently investigated biomarkers are associated with collagen metabolism in cartilage or bone, or aggrecan metabolism in cartilage. Other biomarkers are related to non-collagenous proteins, inflammation and/or fibrosis. Biomarkers in osteoarthritis can be categorised using the burden of disease, investigative, prognostic, efficacy of intervention, diagnostic and safety classification. There are a number of promising candidates, notably urinary C-terminal telopeptide of collagen type II and serum cartilage oligomeric protein, although none is sufficiently discriminating to differentiate between individual patients and controls (diagnostic) or between patients with different disease severities (burden of disease), predict prognosis in individuals with or without osteoarthritis (prognostic) or perform so consistently that it could function as a surrogate outcome in clinical trials (efficacy of intervention). Future avenues for research include exploration of underlying mechanisms of disease and development of new biomarkers; technological development; the 'omics' (genomics, metabolomics, proteomics and lipidomics); design of aggregate scores combining a panel of biomarkers and/or imaging markers into single diagnostic algorithms; and investigation into the relationship between biomarkers and prognosis.
Keywords: Inflammation; Osteoarthritis; Outcomes research.
Figures
Republished in
-
Republished: Value of biomarkers in osteoarthritis: current status and perspectives.Postgrad Med J. 2014 Mar;90(1061):171-8. doi: 10.1136/postgradmedj-2013-203726rep. Postgrad Med J. 2014. PMID: 24534711 Free PMC article.
References
-
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet 2011;377:2115–26 - PubMed
-
- Committee for Medicinal Products for Human Use Guideline on clinical investigation of medicinal products used in the treatment of osteoarthritis. 2010. http://www.ema.europa.eu (accessed 19 Sept 2012)
-
- Food and Drug Administration Guidance for industry. Clinical development programs for drugs, devices, and biological products intended for the treatment of osteoarthritis. 2011. http://www.fda.gov (accessed 12 Jun 2012)
-
- Hunter DJ, Guermazi A. Imaging techniques in osteoarthritis. PM&R 2012;4:S68–74 - PubMed
-
- Atkinson AJ, Colburn WA, DeGruttola VG, et al. Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89–95 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

