Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression
- PMID: 23897811
- PMCID: PMC3784715
- DOI: 10.1074/jbc.M113.498758
Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression
Abstract
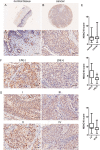
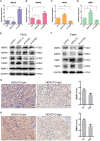
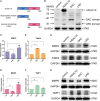
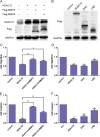
Aberrant expression of histone deacetylases (HDACs) is associated with carcinogenesis. Some HDAC inhibitors are widely considered as promising anticancer therapeutics. A major obstacle for development of HDAC inhibitors as highly safe and effective anticancer therapeutics is that our current knowledge on the contributions of different HDACs in various cancer types remains scant. Here we report that the expression level of HDAC10 was significantly lower in patients exhibiting lymph node metastasis compared with that in patients lacking lymph node metastasis in human cervical squamous cell carcinoma. Forced expression of HDAC10 in cervical cancer cells significantly inhibited cell motility and invasiveness in vitro and metastasis in vivo. Mechanistically, HDAC10 suppresses expression of matrix metalloproteinase (MMP) 2 and 9 genes, which are known to be critical for cancer cell invasion and metastasis. At the molecular level, HDAC10 binds to MMP2 and -9 promoter regions, reduces the histone acetylation level, and inhibits the binding of RNA polymerase II to these regions. Furthermore, an HDAC10 mutant lacking histone deacetylase activity failed to mimic the functions of full-length protein. These results identify a critical role of HDAC10 in suppression of cervical cancer metastasis, underscoring the importance of developing isoform-specific HDAC inhibitors for treatment of certain cancer types such as cervical squamous cell carcinoma.
Keywords: Cell Invasion; Cell Migration; Histone Deacetylase; Matrix Metalloproteinase (MMP); Metastasis.
Figures


References
-
- The Merck Manual Home Health Handbook (2013) Cervical Cancer, www.merckmanuals.com/home/womens_health_issues/cancers_of_the_female_rep...
-
- Armenian Medical Network (2013) Cervical Cancer, www.health.am/cr/cervical-cancer/
-
- Boothello R. (2011) Matrix Metalloproteinases: Its Implications in Cardiovascular Disorders, pharmaxchange.info/press/2011/11/matrix-metalloproteinases-its-implicati...
-
- Yu G., Li H., Wang X., Wu T., Zhu J., Huang S., Wan Y., Tang J. (2013) MicroRNA-19a targets tissue factor to inhibit colon cancer cells migration and invasion. Mol. Cell. Biochem. 380, 239–247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

