Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity
- PMID: 23898034
- PMCID: PMC3723630
- DOI: 10.1105/tpc.113.112409
Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity
Abstract
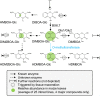
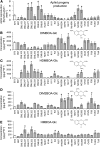
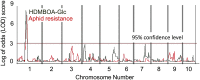
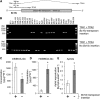
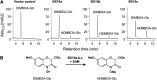
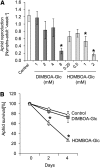
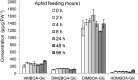
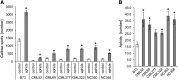
Plants differ greatly in their susceptibility to insect herbivory, suggesting both local adaptation and resistance tradeoffs. We used maize (Zea mays) recombinant inbred lines to map a quantitative trait locus (QTL) for the maize leaf aphid (Rhopalosiphum maidis) susceptibility to maize Chromosome 1. Phytochemical analysis revealed that the same locus was also associated with high levels of 2-hydroxy-4,7-dimethoxy-1,4-benzoxazin-3-one glucoside (HDMBOA-Glc) and low levels of 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside (DIMBOA-Glc). In vitro enzyme assays with candidate genes from the region of the QTL identified three O-methyltransferases (Bx10a-c) that convert DIMBOA-Glc to HDMBOA-Glc. Variation in HDMBOA-Glc production was attributed to a natural CACTA family transposon insertion that inactivates Bx10c in maize lines with low HDMBOA-Glc accumulation. When tested with a population of 26 diverse maize inbred lines, R. maidis produced more progeny on those with high HDMBOA-Glc and low DIMBOA-Glc. Although HDMBOA-Glc was more toxic to R. maidis than DIMBOA-Glc in vitro, BX10c activity and the resulting decline of DIMBOA-Glc upon methylation to HDMBOA-Glc were associated with reduced callose deposition as an aphid defense response in vivo. Thus, a natural transposon insertion appears to mediate an ecologically relevant trade-off between the direct toxicity and defense-inducing properties of maize benzoxazinoids.
Figures


References
-
- Bercury S.D., Panavas T., Irenze K., Walker E.L. (2001). Molecular analysis of the Doppia transposable element of maize. Plant Mol. Biol. 47: 341–351 - PubMed
-
- Botha C.E.J., Matsiliza B. (2004). Reduction in transport in wheat (Triticum aestivum) is caused by sustained phloem feeding by the Russian wheat aphid (Diuraphis noxia). S. Afr. J. Bot. 70: 249–254
-
- Brooks T.D., Willcox M.C., Williams W.P., Buckley P.M. (2005). Quantitative trait loci conferring resistance to fall armyworm and southwestern corn borer leaf feeding damage. Crop Sci. 45: 2430–2434
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

