Tpl2 kinase regulates FcγR signaling and immune thrombocytopenia in mice
- PMID: 23898046
- PMCID: PMC3774848
- DOI: 10.1189/jlb.0113039
Tpl2 kinase regulates FcγR signaling and immune thrombocytopenia in mice
Abstract
The MAPK3 Tpl2 controls innate and adaptive immunity by regulating TLR, TNF-α, and GPCR signaling in a variety of cell types. Its ablation gives rise to an anti-inflammatory phenotype characterized by resistance to LPS-induced endotoxin shock, DSS-induced colitis, and TNF-α-induced IBD. Here, we address the role of Tpl2 in autoimmunity. Our data show that the ablation and the pharmacological inhibition of Tpl2 protect mice from antiplatelet antibody-induced thrombocytopenia, a model of ITP. Thrombocytopenia in this model and in ITP is caused by phagocytosis of platelets opsonized with antiplatelet antibodies and depends on FcγR activation in splenic and hepatic myeloid cells. Further studies explained how Tpl2 inhibition protects from antibody-induced thrombocytopenia, by showing that Tpl2 is activated by FcγR signals in macrophages and that its activation by these signals is required for ERK activation, cytoplasmic Ca(2+) influx, the induction of cytokine and coreceptor gene expression, and phagocytosis.
Keywords: MAPK; immune complex; macrophages.
Figures
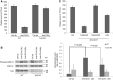

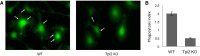
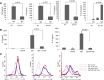
References
-
- Nimmerjahn F., Ravetch J. V. (2008) Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 - PubMed
-
- Salmon J. E., Pricop L. (2001) Human receptors for immunoglobulin G: key elements in the pathogenesis of rheumatic disease. Arthritis Rheum. 44, 739–750 - PubMed
-
- Clynes R., Dumitru C., Ravetch J. V. (1998) Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science 279, 1052–1054 - PubMed
-
- Ji H., Ohmura K., Mahmood U., Lee D. M., Hofhuis F. M., Boackle S. A., Takahashi K., Holers V. M., Walport M., Gerard C., Ezekowitz A., Carroll M. C., Brenner M., Weissleder R., Verbeek J. S., Duchatelle V., Degott C., Benoist C., Mathis D. (2002) Arthritis critically dependent on innate immune system players. Immunity 16, 7–168 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

