Macrophage migration arrest due to a winning balance of Rac2/Sp1 repression over β-catenin-induced PLD expression
- PMID: 23898047
- PMCID: PMC3800072
- DOI: 10.1189/jlb.0313174
Macrophage migration arrest due to a winning balance of Rac2/Sp1 repression over β-catenin-induced PLD expression
Abstract
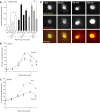
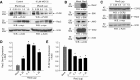
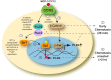
Monocytes and neutrophils infiltrate into tissues during inflammation and stay for extended periods of time until the initial insult is resolved or sometimes remain even longer in the case of chronic inflammation. The mechanism as to why phagocytes become immobilized after the initial cell migration event is not understood completely. Here, we show that overexpression or hyperactivation of Rac2 decreases sustained chemotactic responses of macrophages to MCP-1/CCL2. The resulting leukocyte arrest is not caused by a diminished availability of the cytokine receptor CCR2 that remains intact during MCP-1 stimulation. We show a novel mechanism that links the Rac2-dependent arrest of chemotaxis to decreased expression of PLD2 through the transcription regulator Sp1. Prolonged Rac2 activity leads to nuclear overactivation of Sp1, which acts as a repressor for PLD2. Also, another signaling component plays a regulatory role: β-catenin. Although early times of stimulation (≈ 20 min) with MCP-1/CCL2 resulted in activation of β-catenin with a positive effect on PLD2, after ≈ 3 h of stimulation, the levels of β-catenin were reduced and not able to prevent the negative effect of Rac2 on PLD2 activity. This is a novel molecular mechanism underlying immobilization of monocyte/macrophage migration that is important for the physiological maintenance of leukocytes at the site of inflammation. If this immobilization is prolonged enough, it could lead to chronic inflammation.
Keywords: cell migration; chemotaxis; gene expression regulation; granulocyte; monocyte signaling cascade.
Figures



References
-
- Koshland D. E. (1982) Special topic: chemotaxis and motility. Annu. Rev. Physiol. 44, 499–500
-
- Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709 - PubMed
-
- Vicente-Manzanares M., Webb D. J., Horwitz A. R. (2005) Cell migration at a glance. J. Cell Sci. 118, 4917–4919 - PubMed
-
- Luster A. D., Alon R., von Andrian U. H. (2005) Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182–1190 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

