In vivo effects of anacetrapib on preβ HDL: improvement in HDL remodeling without effects on cholesterol absorption
- PMID: 23898048
- PMCID: PMC3770098
- DOI: 10.1194/jlr.M041541
In vivo effects of anacetrapib on preβ HDL: improvement in HDL remodeling without effects on cholesterol absorption
Abstract
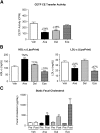
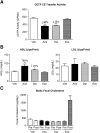
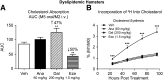
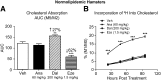
Cholesteryl ester transfer protein (CETP) transfers cholesteryl ester and triglyceride between HDL and apoB-containing lipoproteins. Anacetrapib (ANA), a reversible inhibitor of CETP, raises HDL cholesterol and lowers LDL cholesterol in dyslipidemic patients. We previously demonstrated that ANA increases macrophage-to-feces reverse cholesterol transport and fecal cholesterol excretion in hamsters, and increased preβ HDL-dependent cholesterol efflux via ABCA1 in vitro. However, the effects of ANA on in vivo preβ HDL have not been characterized. In vitro, ANA inhibited the formation of preβ, however in ANA-treated dyslipidemic hamsters, preβ HDL levels (measured by two-dimensional gel electrophoresis) were increased, in contrast to in vitro findings. Because changes in plasma preβ HDL have been proposed to potentially affect markers of cholesterol absorption with other CETP inhibitors, a dual stable isotope method was used to directly measure cholesterol absorption in hamsters. ANA treatment of hamsters (on either dyslipidemic or normal diet) had no effect on cholesterol absorption, while dalcetrapib-treated hamsters displayed an increase in cholesterol absorption. Taken together, these data support the notion that ANA promotes preβ HDL functionality in vivo, with no effects on cholesterol absorption.
Keywords: anacetrapib; apolipoprotein A1; cholesteryl ester transfer protein; dalcetrapib; high density lipoprotein.
Figures


References
-
- Davidson M. H., McKenney J. M., Shear C. L., Revkin J. H. 2006. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels. J. Am. Coll. Cardiol. 48: 1774–1781 - PubMed
-
- McKenney J. M., Davidson M. H., Shear C. L., Revkin J. H. 2006. Efficacy and safety of torcetrapib, a novel cholesteryl ester transfer protein inhibitor, in individuals with below-average high-density lipoprotein cholesterol levels on a background of atorvastatin. J. Am. Coll. Cardiol. 48: 1782–1790 - PubMed
-
- Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J. P., Komajda M., Lopez-Sendon J., Mosca L., Tardif J., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 - PubMed
-
- Forrest M. J., Bloomfield D., Briscoe R. J., Brown R. N., Cumiskey A. M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154: 1465–1473 - PMC - PubMed
-
- DePasquale M., Cadelina G., Knight D., Loging W., Winter S., Blasi E., Perry D., Keiser J. 2009. Mechanistic studies of blood pressure in rats treated with a series of cholesteryl ester transfer protein inhibitors. Drug Dev. Res. 70: 35–48
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

