Source-to-sink transport of sugar and regulation by environmental factors
- PMID: 23898339
- PMCID: PMC3721551
- DOI: 10.3389/fpls.2013.00272
Source-to-sink transport of sugar and regulation by environmental factors
Abstract
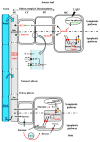
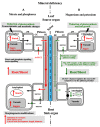
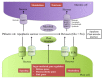
Source-to-sink transport of sugar is one of the major determinants of plant growth and relies on the efficient and controlled distribution of sucrose (and some other sugars such as raffinose and polyols) across plant organs through the phloem. However, sugar transport through the phloem can be affected by many environmental factors that alter source/sink relationships. In this paper, we summarize current knowledge about the phloem transport mechanisms and review the effects of several abiotic (water and salt stress, mineral deficiency, CO2, light, temperature, air, and soil pollutants) and biotic (mutualistic and pathogenic microbes, viruses, aphids, and parasitic plants) factors. Concerning abiotic constraints, alteration of the distribution of sugar among sinks is often reported, with some sinks as roots favored in case of mineral deficiency. Many of these constraints impair the transport function of the phloem but the exact mechanisms are far from being completely known. Phloem integrity can be disrupted (e.g., by callose deposition) and under certain conditions, phloem transport is affected, earlier than photosynthesis. Photosynthesis inhibition could result from the increase in sugar concentration due to phloem transport decrease. Biotic interactions (aphids, fungi, viruses…) also affect crop plant productivity. Recent breakthroughs have identified some of the sugar transporters involved in these interactions on the host and pathogen sides. The different data are discussed in relation to the phloem transport pathways. When possible, the link with current knowledge on the pathways at the molecular level will be highlighted.
Keywords: Phloem; abiotic factors; biotic factors; source/sink; sugar transport.
Figures
References
-
- Abbes Z., Kharrat M., Delavault P., Chaïbi W., Simier P. (2009a) Nitrogen and carbon relationships between the parasitic weed Orobanche foetida and susceptible and tolerant faba bean lines. Plant Physiol. Biochem. 47 153–159 - PubMed
-
- Agasse A., Vignault C., Kappel C., Conde C., Gerós H., Delrot S. (2009) “Sugar transport and sugar sensing in grape,” in Grapevine Molecular Physiology and Biotechnology ed. Roubelakis-Angelakis K.A. (Netherland: Springer; ) 105–139
LinkOut - more resources
Full Text Sources
Other Literature Sources

