Strategies to increase drug penetration in solid tumors
- PMID: 23898462
- PMCID: PMC3724174
- DOI: 10.3389/fonc.2013.00193
Strategies to increase drug penetration in solid tumors
Abstract
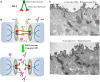
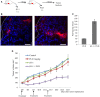
Despite significant improvement in modalities for treatment of cancer that led to a longer survival period, the death rate of patients with solid tumors has not changed during the last decades. Emerging studies have identified several physical barriers that limit the therapeutic efficacy of cancer therapeutic agents such as monoclonal antibodies, chemotherapeutic agents, anti-tumor immune cells, and gene therapeutics. Most solid tumors are of epithelial origin and, although malignant cells are de-differentiated, they maintain intercellular junctions, a key feature of epithelial cells, both in the primary tumor as well as in metastatic lesions. Furthermore, nests of malignant epithelial tumor cells are shielded by layers of extracellular matrix (ECM) proteins (e.g., collagen, elastin, fibronectin, laminin) whereby tumor vasculature rarely penetrates into the tumor nests. In this chapter, we will review potential strategies to modulate the ECM and epithelial junctions to enhance the intratumoral diffusion and/or to remove physical masking of target receptors on malignant cells. We will focus on peptides that bind to the junction protein desmoglein 2 and trigger intracellular signaling, resulting in the transient opening of intercellular junctions. Intravenous injection of these junction openers increased the efficacy and safety of therapies with monoclonal antibodies, chemotherapeutics, and T cells in mouse tumor models and was safe in non-human primates. Furthermore, we will summarize approaches to transiently degrade ECM proteins or downregulate their expression. Among these approaches is the intratumoral expression of relaxin or decorin after adenovirus- or stem cell-mediated gene transfer. We will provide examples that relaxin-based approaches increase the anti-tumor efficacy of oncolytic viruses, monoclonal antibodies, and T cells.
Keywords: epithelial junctions; extracellular matrix; junction opener; relaxin; tumor stroma; tumor-associated macrophages.
Figures



Similar articles
-
Overcoming physical barriers in cancer therapy.Tissue Barriers. 2013 Jan 1;1(1):e23647. doi: 10.4161/tisb.23647. Tissue Barriers. 2013. PMID: 24665377 Free PMC article.
-
Epithelial Junction Opener Improves Oncolytic Adenovirus Therapy in Mouse Tumor Models.Hum Gene Ther. 2016 Apr;27(4):325-37. doi: 10.1089/hum.2016.022. Hum Gene Ther. 2016. PMID: 26993072 Free PMC article.
-
Controlled extracellular matrix degradation in breast cancer tumors improves therapy by trastuzumab.Mol Ther. 2011 Mar;19(3):479-89. doi: 10.1038/mt.2010.256. Epub 2010 Nov 16. Mol Ther. 2011. PMID: 21081901 Free PMC article.
-
CD44: structure, function, and association with the malignant process.Adv Cancer Res. 1997;71:241-319. doi: 10.1016/s0065-230x(08)60101-3. Adv Cancer Res. 1997. PMID: 9111868 Review.
-
Strategies for enhancing intratumoral spread of oncolytic adenoviruses.Pharmacol Ther. 2020 Sep;213:107586. doi: 10.1016/j.pharmthera.2020.107586. Epub 2020 May 30. Pharmacol Ther. 2020. PMID: 32479843 Review.
Cited by
-
Preclinical safety and efficacy studies with an affinity-enhanced epithelial junction opener and PEGylated liposomal doxorubicin.Mol Ther Methods Clin Dev. 2015 Mar 11;2:15005. doi: 10.1038/mtm.2015.5. eCollection 2015. Mol Ther Methods Clin Dev. 2015. PMID: 26029716 Free PMC article.
-
Modulation of extracellular matrix in cancer is associated with enhanced tumor cell targeting by bacteriophage vectors.Mol Cancer. 2015 Jun 3;14:110. doi: 10.1186/s12943-015-0383-4. Mol Cancer. 2015. PMID: 26037383 Free PMC article.
-
Extracellular matrix macromolecules: potential tools and targets in cancer gene therapy.Mol Cell Ther. 2014 May 2;2:14. doi: 10.1186/2052-8426-2-14. eCollection 2014. Mol Cell Ther. 2014. PMID: 26056582 Free PMC article. Review.
-
Targeting pancreatic cancer with combinatorial treatment of CPI-613 and inhibitors of lactate metabolism.PLoS One. 2022 Apr 22;17(4):e0266601. doi: 10.1371/journal.pone.0266601. eCollection 2022. PLoS One. 2022. PMID: 35452495 Free PMC article.
-
Cannabidiol on the Path from the Lab to the Cancer Patient: Opportunities and Challenges.Pharmaceuticals (Basel). 2022 Mar 17;15(3):366. doi: 10.3390/ph15030366. Pharmaceuticals (Basel). 2022. PMID: 35337163 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

