Ion channel models based on self-assembling cyclic peptide nanotubes
- PMID: 23898935
- PMCID: PMC3867521
- DOI: 10.1021/ar400061d
Ion channel models based on self-assembling cyclic peptide nanotubes
Abstract
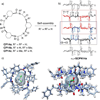
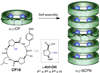
The lipid bilayer membranes are Nature's dynamic structural motifs that individualize cells and keep ions, proteins, biopolymers and metabolites confined in the appropriate location. The compartmentalization and isolation of these molecules from the external media facilitate the sophisticated functions and connections between the different biological processes accomplished by living organisms. However, cells require assistance from minimal energy shortcuts for the transport of molecules across membranes so that they can interact with the exterior and regulate their internal environments. Ion channels and pores stand out from all other possible transport mechanisms due to their high selectivity and efficiency in discriminating and transporting ions or molecules across membrane barriers. Nevertheless, the complexity of these smart "membrane holes" has driven researchers to develop simpler artificial structures with comparable performance to the natural systems. As a broad range of supramolecular interactions have emerged as efficient tools for the rational design and preparation of stable 3D superstructures, these results have stimulated the creativity of chemists to design synthetic mimics of natural active macromolecules and even to develop artificial structures with functions and properties. In this Account, we highlight results from our laboratories on the construction of artificial ion channel models that exploit the self-assembly of conformationally flat cyclic peptides (CPs) into supramolecular nanotubes. Because of the straightforward synthesis of the cyclic peptide monomers and the complete control over the internal diameter and external surface properties of the resulting hollow tubular suprastructure, CPs are the optimal candidates for the fabrication of ion channels. The ion channel activity and selective transport of small molecules by these structures are examples of the great potential that cyclic peptide nanotubes show for the construction of functional artificial transmembrane transporters. Our experience to date suggests that the next steps for achieving conceptual devices with better performance and selectivity will derive from the topological control over cyclic peptide assembly and the functionalization of the lumen.
Figures
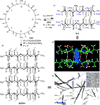
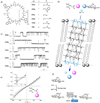
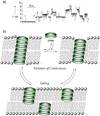

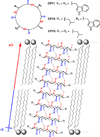

References
-
- Stein WD. Channels, Carriers, and Pumps: An Introduction to Membrane Transport. San Diego, CA: Academic Press; 1990.
-
- Hille B. Ionic Channels of Excitable Membranes. 2nd ed. Sunderland, MA: Sinauer; 1992.
-
- Eisenberg B. Ionic channels in biological membranes: natural nanotubes. Acc. Chem. Res. 1998;31:117–124.
-
- Doyle DA. The Structure of the Potassium Channel: Molecular Basis of K+ Conduction and Selectivity. Science. 1998;280:69–77. - PubMed
-
- de Groot BL, Grubmüller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294:2353–2357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

