An effective multisource informed consent procedure for research and clinical practice: an observational study of patient understanding and awareness of their roles as research stakeholders in a cancer biobank
- PMID: 23899250
- PMCID: PMC3734192
- DOI: 10.1186/1472-6939-14-30
An effective multisource informed consent procedure for research and clinical practice: an observational study of patient understanding and awareness of their roles as research stakeholders in a cancer biobank
Abstract
Background: Efforts to improve patients' understanding of their own medical treatments or research in which they are involved are progressing, especially with regard to informed consent procedures. We aimed to design a multisource informed consent procedure that is easily adaptable to both clinical and research applications, and to evaluate its effectiveness in terms of understanding and awareness, even in less educated patients.
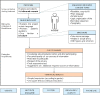
Methods: We designed a multisource informed consent procedure for patients' enrolment in a Cancer Institute Biobank (CRO-Biobank). From October 2009 to July 2011, a total of 550 cancer patients admitted to the Centro di Riferimento Oncologico IRCCS Aviano, who agreed to contribute to its biobank, were consecutively enrolled. Participants were asked to answer a self-administered questionnaire aim at exploring their understanding of biobanks and their needs for information on this topic, before and after study participation. Chi-square tests were performed on the questionnaire answers, according to gender or education.
Results: Of the 430 patients who returned the questionnaire, only 36.5% knew what a biobank was before participating in the study. Patients with less formal education were less informed by some sources (the Internet, newspapers, magazines, and our Institute). The final assessment test, taken after the multisource informed consent procedure, showed more than 95% correct answers. The information received was judged to be very or fairly understandable in almost all cases. More than 95% of patients were aware of participating in a biobank project, and gave helping cancer research (67.5%), moral obligation, and supporting cancer care as main reasons for their involvement.
Conclusions: Our multisource informed consent information system allowed a high rate of understanding and awareness of study participation, even among less-educated participants, and could be an effective and easy-to-apply model for others to consider to contribute to a well-informed decision making process in several fields, from clinical practice to research.Further studies are needed to explore the effects on the study comprehension by each source of information, and by other sources suggested by participants in the questionnaire.
Figures
Similar articles
-
The effectiveness of health literacy interventions on the informed consent process of health care users: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):82-94. doi: 10.11124/jbisrir-2015-2304. JBI Database System Rev Implement Rep. 2015. PMID: 26571285
-
Research participants' perceptions and views on consent for biobank research: a review of empirical data and ethical analysis.BMC Med Ethics. 2015 Sep 9;16:60. doi: 10.1186/s12910-015-0053-5. BMC Med Ethics. 2015. PMID: 26354520 Free PMC article. Review.
-
Exploring Understanding of "Understanding": The Paradigm Case of Biobank Consent Comprehension.Am J Bioeth. 2019 May;19(5):6-18. doi: 10.1080/15265161.2019.1587031. Am J Bioeth. 2019. PMID: 31068107 Free PMC article.
-
Recall and Retention of Consent Procedure Contents and Decisions: Results of a Randomized Controlled Trial.Public Health Genomics. 2018;21(1-2):27-36. doi: 10.1159/000492662. Epub 2018 Sep 10. Public Health Genomics. 2018. PMID: 30199881 Clinical Trial.
-
Participants' Understanding of Informed Consent for Biobanking: A Systematic Review.Clin Nurs Res. 2019 Jan;28(1):30-51. doi: 10.1177/1054773817722690. Epub 2017 Jul 26. Clin Nurs Res. 2019. PMID: 28745067
Cited by
-
Electronic Video Consent to Power Precision Health Research: A Pilot Cohort Study.JMIR Form Res. 2021 Sep 8;5(9):e29123. doi: 10.2196/29123. JMIR Form Res. 2021. PMID: 34313247 Free PMC article.
-
How Semantics Connotations May Influence Concerns About Donation of Biospecimens.Biopreserv Biobank. 2021 Jun;19(3):156-162. doi: 10.1089/bio.2020.0072. Epub 2020 Nov 11. Biopreserv Biobank. 2021. PMID: 33179960 Free PMC article.
-
Genetic and ElectroNic medIcal records to predict oUtcomeS in Heart Failure patients (GENIUS-HF) - design and rationale.BMC Cardiovasc Disord. 2014 Mar 4;14:32. doi: 10.1186/1471-2261-14-32. BMC Cardiovasc Disord. 2014. PMID: 24592820 Free PMC article.
-
Attitudes of oncology patients' towards biospecimen donation for biobank research.BMC Cancer. 2024 Mar 27;24(1):390. doi: 10.1186/s12885-024-12145-5. BMC Cancer. 2024. PMID: 38539134 Free PMC article.
-
BNC2 is a putative tumor suppressor gene in high-grade serous ovarian carcinoma and impacts cell survival after oxidative stress.Cell Death Dis. 2016 Sep 22;7(9):e2374. doi: 10.1038/cddis.2016.278. Cell Death Dis. 2016. PMID: 27899818 Free PMC article.
References
-
- Toccaceli V, Fagnani C, Nistico L, D'Ippolito C, Giannantonio L, Brescianini S, Stazi MA. Research understanding, attitude and awareness towards biobanking: a survey among Italian twin participants to a genetic epidemiological study. BMC Med Ethics. 2009;10:4. doi: 10.1186/1472-6939-10-4. - DOI - PMC - PubMed
-
- . Nuremberg Code. 2012. http://www.hhs.gov/ohrp/archive/nurcode.html.
-
- 18th WMA General Assembly. World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 2012. http://science.education.nih.gov/supplements/nih9/bioethics/guide/teache.... - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

